Abstract
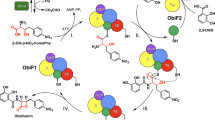
Saframycin A is a potent antitumor antibiotic with a unique pentacyclic tetrahydroisoquinoline scaffold. We found that the nonribosomal peptide synthetase SfmC catalyzes a seven-step transformation of readily synthesized dipeptidyl substrates with long acyl chains into a complex saframycin scaffold. Based on a series of enzymatic reactions, we propose a detailed mechanism involving the reduction of various peptidyl thioesters by a single R domain followed by iterative C domain–mediated Pictet-Spengler reactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Arai, T., Takahashi, K. & Kubo, A. J. Antibiot. (Tokyo) 30, 1015–1018 (1977).
Scott, J.D. & Williams, R.M. Chem. Rev. 102, 1669–1730 (2002).
Cuevas, C. & Francesch, A. Nat. Prod. Rep. 26, 322–337 (2009).
Cuevas, C. et al. Org. Lett. 2, 2545–2548 (2000).
Watanabe, K. et al. Nat. Chem. Biol. 2, 423–428 (2006).
Watanabe, K. et al. J. Am. Chem. Soc. 131, 9347–9353 (2009).
Watanabe, K. et al. ChemBioChem 10, 1965–1968 (2009).
Mikami, Y. et al. J. Biol. Chem. 260, 344–348 (1985).
Velasco, A. et al. Mol. Microbiol. 56, 144–154 (2005).
Fu, C.Y. et al. J. Microbiol. Biotechnol. 19, 439–446 (2009).
Pospiech, A., Bietenhader, J. & Schupp, T. Microbiology 142, 741–746 (1996).
Li, L. et al. J. Bacteriol. 190, 251–263 (2008).
Schmidt, E.W., Nelson, J.T. & Fillmore, J.P. Tetrahedr. Lett. 45, 3921–3924 (2004).
Fischbach, M.A. & Walsh, C.T. Chem. Rev. 106, 3468–3496 (2006).
Baltz, R.H., Miao, V. & Wrigley, S.K. Nat. Prod. Rep. 22, 717–741 (2005).
Hansen, D.B., Bumpus, S.B., Aron, Z.D., Kelleher, N.L. & Walsh, C.T. J. Am. Chem. Soc. 129, 6366–6367 (2007).
Du, L., Sanchez, C., Chen, M., Edwards, D.J. & Shen, B. Chem. Biol. 7, 623–642 (2000).
Mossialos, D. et al. Mol. Microbiol. 45, 1673–1685 (2002).
Visca, P., Imperi, F. & Lamont, I.L. Trends Microbiol. 15, 22–30 (2007).
Xue, Y. & Sherman, D.H. Nature 403, 571–575 (2000).
Myers, A.G. & Kung, D.W. J. Am. Chem. Soc. 121, 10828–10829 (1999).
Bennett, B.D. et al. Nat. Chem. Biol. 5, 593–599 (2009).
Duerfahrt, T., Eppelmann, K., Muller, R. & Marahiel, M.A. Chem. Biol. 11, 261–271 (2004).
Acknowledgements
This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) ((B)20310126 to H. Oikawa) and in part by a grant from Japan Science and Technology (JST) (Research for Promoting Technological Seeds 01-073 to H. Oikawa). A fellowship to K.K. from the JSPS is gratefully acknowledged. We thank Y. Kumaki and S. Oka (both of Hokkaido University) for obtaining NMR and mass data.
Author information
Authors and Affiliations
Contributions
H. Oikawa and K.W. designed the research, and H. Oikawa wrote the manuscript. K.K. and H.S. synthesized the substrates for the enzymatic reactions. K.W. cloned the sfmC gene and optimized the expression conditions. K.K. prepared the deletion enzymes and performed all enzymatic reactions. H. Oguri advised on the research and made a significant contribution to a proposal of the reaction mechanism.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Figures 1–17 and Supplementary Tables 1 and 2 (PDF 1761 kb)
Rights and permissions
About this article
Cite this article
Koketsu, K., Watanabe, K., Suda, H. et al. Reconstruction of the saframycin core scaffold defines dual Pictet-Spengler mechanisms. Nat Chem Biol 6, 408–410 (2010). https://doi.org/10.1038/nchembio.365
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.365
This article is cited by
-
Chemoenzymatic approaches for exploring structure–activity relationship studies of bioactive natural products
Nature Synthesis (2023)
-
Reductive inactivation of the hemiaminal pharmacophore for resistance against tetrahydroisoquinoline antibiotics
Nature Communications (2021)
-
The hidden enzymology of bacterial natural product biosynthesis
Nature Reviews Chemistry (2019)
-
Enzyme catalysed Pictet-Spengler formation of chiral 1,1’-disubstituted- and spiro-tetrahydroisoquinolines
Nature Communications (2017)
-
In vivo investigation of the role of SfmO2 in saframycin A biosynthesis by structural characterization of the analogue saframycin O
Science China Chemistry (2012)