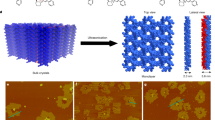
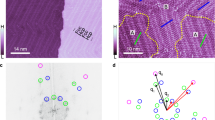
Abstract
The chirality of molecular structures is paramount in many phenomena, including enantioselective reactions, molecular self-assembly, biological processes and light or electron-spin polarization. Flat prochiral molecules, which are achiral in the gas phase or solution, can exhibit adsorption-induced chirality when deposited on surfaces. The whole array of such molecular adsorbates is naturally racemic as spontaneous global mirror-symmetry breaking is disfavoured. Here we demonstrate a chemical method of obtaining flat prochiral molecules adsorbed on the solid achiral surface in such a way that a specific adsorbate handedness globally dominates. An optically pure helical precursor is flattened in a cascade of on-surface reactions, which enables chirality transfer. The individual reaction products are identified by high-resolution scanning-probe microscopy. The ultimate formation of globally non-racemic assemblies of flat molecules through stereocontrolled on-surface synthesis allows for chirality to be expressed in as yet unexplored types of organic–inorganic chiral interfaces.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blackmond, D. G. The origin of biological homochirality. Cold Spring Harb. Perspect. Biol. 2, a002147 (2010).
Carreira, E. M. & Kvaerno, L. Classics in Stereoselective Synthesis (Wiley-VCH, 2009).
Temirov, R., Soubatch, S., Neucheva, O., Lassise, A. C. & Tautz, F. S. A novel method achieving ultra-high geometrical resolution in scanning tunnelling microscopy. New J. Phys. 10, 053012 (2008).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Hapala, P. et al. The mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Gourdon, A. On-surface covalent coupling in ultrahigh vacuum. Angew. Chem. Int. Ed. 47, 6950–6953 (2008).
Méndez, J., López, M. F. & Martín-Gago, J. A. On-surface synthesis of cyclic organic molecules. Chem. Soc. Rev. 40, 4578–4590 (2011).
Zhang, X., Zeng, Q. & Wang, C. On-surface single molecule synthesis chemistry: a promising bottom-up approach towards functional surfaces. Nanoscale 5, 8269–8287 (2013).
de Oteyza, D. G. et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 340, 1434–1437 (2013).
Pavliček, N. et al. On-surface generation and imaging of arynes by atomic force microscopy. Nat. Chem. 7, 623–628 (2015).
Demers-Carpentier, V. et al. Stereodirection of an α-ketoester at sub-molecular sites on chirally modified Pt(111): heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 135, 9999–10002 (2013).
Amabilino, D. B. Chirality at the Nanoscale (Wiley-VCH, 2009).
Barlow, S. M. & Raval, R. Complex organic molecules at metal surfaces: bonding, organisation and chirality. Surf. Sci. Rep. 50, 201–341 (2003).
Ernst, K.-H. Molecular chirality at surfaces. Phys. Status Solidi B 249, 2057–2088 (2012).
Eckhardt, C. J. et al. Separation of chiral phases in monolayer crystals of racemic amphiphiles. Nature 362, 614–616 (1993).
Stöhr, M. et al. Self-assembly and two-dimensional spontaneous resolution of cyano-functionalized [7]helicenes on Cu(111). Angew. Chem. Int. Ed. 50, 9982–9986 (2011).
Blüm, M.-C., Ćavar, E., Pivetta, M., Patthey, F. & Schneider, W.-D. Conservation of chirality in a hierarchical supramolecular self-assembled structure with pentagonal symmetry. Angew. Chem. Int. Ed. 44, 5334–5337 (2005).
Sun, K. et al. Chiral pinwheel clusters lacking local point chirality. Small 8, 2078–2082 (2012).
Parschau, M. et al. Buckybowls on metal surfaces: symmetry mismatch and enantiomorphism of corannulene on Cu(110). Angew. Chem. Int. Ed. 46, 8258–8261 (2007).
Fasel, R., Parschau, M. & Ernst, K.-H. Amplification of chirality in two-dimensional enantiomorphous lattices. Nature 439, 449–452 (2006).
Haq, S., Liu, N., Humblot, V., Jansen, A. P. J. & Raval, R. Drastic symmetry breaking in supramolecular organization of enantiomerically unbalanced monolayers at surfaces. Nat. Chem. 1, 409–414 (2009).
Parschau, M., Romer, S. & Ernst, K.-H. Induction of homochirality in achiral enantiomorphous monolayers. J. Am. Chem. Soc. 126, 15398–15399 (2004).
Destoop, I. et al. ‘Sergeants-and-Corporals’ principle in chiral induction at an interface. Chem. Commun. 49, 7477–7479 (2013).
Kündig, P. E. Transition Metal Arene π-Complexes in Organic Synthesis and Catalysis (Springer, 2004).
Dai, L.-X. et al. Chiral Ferrocenes in Asymmetric Catalysis (Wiley-VCH, 2010).
Gleiter, R. et al. Modern Cyclophane Chemistry (Wiley-VCH, 2004).
Jančařík, A. et al. Rapid access to dibenzohelicenes and their functionalized derivatives. Angew. Chem. Int. Ed. 52, 9970–9975 (2013).
Pinardi, A. L. et al. Tailored formation of N-doped nanoarchitectures by diffusion-controlled on-surface (cyclo)dehydrogenation of heteroaromatics. ACS Nano 7, 3676–3684 (2013).
Pinardi, A. L. et al. Sequential formation of N-doped nanohelicenes, nanographenes and nanodomes by surface-assisted chemical (cyclo)dehydrogenation of heteroaromatics. Chem. Commun. 50, 1555–1557 (2014).
Seibel, J., Parschau, M. & Ernst, K.-H. Two-dimensional crystallization of enantiopure and racemic heptahelicene on Ag(111) and Au(111). J. Phys. Chem. C 118, 29135–29141 (2014).
Seibel, J., Allemann, O., Siegel, J. S. & Ernst, K.-H. Chiral conflict among different helicenes suppresses formation of one enantiomorph in 2D crystallization. J. Am. Chem. Soc. 135, 7434–7437 (2013).
Wang, D. Z., Katz, T. J., Golen, J. & Rheingold, A. L. Diels–Alder additions of benzynes within helicene skeletons. J. Org. Chem. 69, 7769–7771 (2004).
Groen, M. B., Schadenberg, H. & Wynberg, H. Synthesis and resolution of some heterohelicenes. J. Org. Chem. 36, 2797–2809 (1971).
Fuchter, M. J., Weimar, M., Yang, X., Judge, D. K. & White, A. J. P. An unusual oxidative rearrangement of [7]-helicene. Tetrahedron Lett. 53, 1108–1111 (2012).
Berger, R. J. F. et al. Gold(I) mediated rearrangement of [7]-helicene to give a benzo[cd]pyrenium cation embedded in a chiral framework. Chem. Commun. 50, 5251–5253 (2014).
Kichin, G., Weiss, C., Wagner, C., Tautz, F. S. & Temirov, R. Single molecule and single atom sensors for atomic resolution imaging of chemically complex surfaces. J. Am. Chem. Soc. 133, 16847–16851 (2011).
Acknowledgements
This work was financially supported by a Czech Science Foundation grant (14-29667S, 14-16963J) and by the Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences (RVO: 61388963).
Author information
Authors and Affiliations
Contributions
P.J. and I.S. conceived the project and designed the experiments. O.S. and M.Š. performed and analysed the SPM experiments. A.J. synthesized racemic DBH, and J.R. resolved the racemic DBH into enantiomers. J.V.C., J.V., K.K. and P.J. performed theoretical calculations and interpreted their results. I.S. and I.G.S. interpreted the chemical transformations. P.J., O.S., M.Š., I.S. and I.G.S. co-wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4640 kb)
Rights and permissions
About this article
Cite this article
Stetsovych, O., Švec, M., Vacek, J. et al. From helical to planar chirality by on-surface chemistry. Nature Chem 9, 213–218 (2017). https://doi.org/10.1038/nchem.2662
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2662
This article is cited by
-
On-surface cyclization of vinyl groups on poly-para-phenylene involving an unusual pentagon to hexagon transformation
Nature Communications (2024)
-
Generating antiaromaticity in polycyclic conjugated hydrocarbons by thermally selective skeletal rearrangements at interfaces
Nature Synthesis (2023)
-
Simultaneous switching of supramolecular chirality and organizational chirality driven by Coulomb expansion
Nano Research (2022)
-
Oxidative cyclo-rearrangement of helicenes into chiral nanographenes
Nature Communications (2021)
-
Assigning the absolute configuration of single aliphatic molecules by visual inspection
Nature Communications (2018)