Abstract
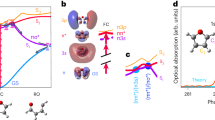
Although the water oxidation cycle involves the critical step of O–O bond formation, the transition metal oxide radical thought to be the catalytic intermediate for this step has eluded direct observation. The radical represents the transformation of charge into a nascent catalytic intermediate, which lacks a newly formed bond and is therefore inherently difficult to detect. Here, using theoretical calculations and ultrafast in situ infrared spectroscopy of photocatalysis at an n-SrTiO3/aqueous interface, we reveal a subsurface vibration of the oxygen directly below, and uniquely generated by, the oxyl radical (Ti–O•). Intriguingly, this interfacial Ti–O stretch vibration, once decoupled from the lattice, couples to reactant dynamics (water librations). These experiments demonstrate subsurface vibrations and their coupling to solvent and electron dynamics to detect nascent catalytic intermediates at the solid–liquid interface at the molecular level. One can envision using the subsurface vibrations and their coupling across the interface to track and control catalysis dynamically.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
18 May 2016
The original version of this Article contained an incorrectly labelled y axis in Fig. 2c. The chart is showing normalized data and so the y axis should have been labelled 'ΔA (a.u.)'. This has been corrected in all versions of the Article.
References
Licht, S. et al. Efficient solar water splitting, exemplified by RuO2-catalyzed AlGaAs/Si photoelectrolysis. J. Phys. Chem. B 104, 8920–8924 (2000).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Eisenberg, R. & Gray, H. B. Preface on making oxygen. Inorg. Chem. 47, 1697–1699 (2008).
Zhang, M., de Respinis, M. & Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nature Chem. 6, 362–367 (2014).
Ramasesha, K., De Marco, L., Mandal, A. & Tokmakoff, A. Water vibrations have strongly mixed intra- and intermolecular character. Nature Chem. 5, 935–940 (2013).
Renger, G. Light induced oxidative water splitting in photosynthesis: energetics, kinetics and mechanism. J. Photochem. Photobiol. B 104, 35–43 (2011).
Yano, J. & Yachandra, V. K. Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster from X-ray spectroscopy. Inorg. Chem. 47, 1711–1726 (2008).
Tagore, R., Crabtree, R. H. & Brudvig, G. W. Oxygen evolution catalysis by a dimanganese complex and its relation to photosynthetic water oxidation. Inorg. Chem. 47, 1815–1823 (2008).
Siegbahn, P. E. M. Theoretical studies of O–O bond formation in photosystem II. Inorg. Chem. 47, 1779–1786 (2008).
Groot, M. L. et al. Initial electron donor and acceptor in isolated photosystem II reaction centers identified with femtosecond mid-IR spectroscopy. Proc. Natl Acad. Sci. USA. 102, 13087–13092 (2005).
Britt, R. D. et al. Recent pulsed EPR studies of the photosystem II oxygen-evolving complex: implications as to water oxidation mechanisms. Biochim. Biophys. Acta 1655, 158–171 (2004).
Kern, J. et al. Taking snapshots of photosynthetic water oxidation using femtosecond X-ray diffraction and spectroscopy. Nature Commun. 5, 4371 (2014).
Cummings, C. Y., Marken, F., Peter, L. M., Wijayantha, K. G. U. & Tahir, A. A. New insights into water splitting at mesoporous α-Fe2O3 films: a study by modulated transmittance and impedance spectroscopies. J. Am. Chem. Soc. 134, 1228–1234 (2012).
Klahr, B. & Hamann, T. Water oxidation on hematite photoelectrodes: insight into the nature of surface states through in situ spectroelectrochemistry. J. Phys. Chem. C 118, 10393–10399 (2014).
Norton, A. P., Bernasek, S. L. & Bocarsly, A. B. Mechanistic aspects of the photooxidation of water at the n-titania/aqueous interface: optically induced transients as a kinetic probe. J. Phys. Chem. 92, 6009–6016 (1988).
Nakamura, R. & Nakato, Y. Primary intermediates of oxygen photoevolution reaction on TiO2 (rutile) particles, revealed by in situ FTIR absorption and photoluminescence measurements. J. Am. Chem. Soc. 126, 1290–1298 (2004).
Imanishi, A., Okamura, T., Ohashi, N., Nakamura, R. & Nakato, Y. Mechanism of water photooxidation reaction at atomically flat TiO2 (rutile) (110) and (100) surfaces: dependence on solution pH. J. Am. Chem. Soc. 129, 11569–11578 (2007).
Micic, O. I., Zhang, Y., Cromack, K. R., Trifunac, A. D. & Thurnauer, M. C. Trapped holes on titania colloids studied by electron paramagnetic resonance. J. Phys. Chem. 97, 7277–7283 (1993).
Kanan, M. W. et al. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J. Am. Chem. Soc. 132, 13692–13701 (2010).
McAlpin, J. G. et al. Electronic structure description of a [Co(III)3Co(IV)O4] cluster: a model for the paramagnetic intermediate in cobalt-catalyzed water oxidation. J. Am. Chem. Soc. 133, 15444–15452 (2011).
Surendranath, Y., Kanan, M. W. & Nocera, D. G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 132, 16501–16509 (2010).
Bediako, D. K., Costentin, C., Jones, E. C., Nocera, D. G. & Savéant, J. Proton–electron transport and transfer in electrocatalytic films. application to a cobalt-based O2-evolution catalyst. J. Am. Chem. Soc. 135, 10492–10502 (2013).
Bediako, D. K., Surendranath, Y. & Nocera, D. G. Mechanistic studies of the oxygen evolution reaction mediated by a nickel-borate thin film electrocatalyst. J. Am. Chem. Soc. 135, 3662–3674 (2013).
Huynh, M., Bediako, D. K. & Nocera, D. G. A functionally stable manganese oxide oxygen evolution catalyst in acid. J. Am. Chem. Soc. 136, 6002–6010 (2014).
Sivasankar, N., Weare, W. W. & Frei, H. Direct observation of a hydroperoxide surface intermediate upon visible light-driven water oxidation at an Ir oxide nanocluster catalyst by rapid-scan FT-IR spectroscopy. J. Am. Chem. Soc. 133, 12976–12979 (2011).
Sezen, H. et al. Evidence for photogenerated intermediate hole polarons in ZnO. Nature Commun. 6, 6901 (2015).
Sezen, H. et al. Probing electrons in TiO2 polaronic trap states by IR-absorption: evidence for the existence of hydrogenic states. Sci. Rep. 4, 3808 (2014).
Waegele, M. M., Chen, X., Herlihy, D. M. & Cuk, T. How surface potential determines the kinetics of the first hole transfer of photocatalytic water oxidation. J. Am. Chem. Soc. 136, 10632–10639 (2014).
Kasinski, J. J., Gomez-Jahn, L. A., Faran, K. J., Gracewski, S. M. & Miller, R. J. D. Picosecond dynamics of surface electron transfer processes: surface restricted transient grating studies of the n-TiO2/H2O interface. J. Chem. Phys. 90, 1253–1269 (1989).
Lane, I. M., King, D. A., Liu, Z. & Arnolds, H. Real-time observation of nonadiabatic surface dynamics: the first picosecond in the dissociation of NO on iridium. Phys. Rev. Lett. 97, 186105 (2006).
Haran, G., Sun, W. D., Wynne, K. & Hochstrasser, R. M. Femtosecond far-infrared pump-probe spectroscopy: a new tool for studying low-frequency vibrational dynamics in molecular condensed phases. Chem. Phys. Lett. 274, 365–371 (1997).
Roberts, S. T., Ramasesha, K., Petersen, P. B., Mandal, A. & Tokmakoff, A. Proton transfer in concentrated aqueous hydroxide visualized using ultrafast infrared spectroscopy. J. Phys. Chem. A 115, 3957–3972 (2011).
Bakker, H., Bonn, M. & Fayer, M. Femtosecond Vibrational Spectroscopy of Aqueous Systems (CRC, 2013).
Hussain, H. et al. A quantitative structural investigation of the 0.1 wt % Nb-SrTiO3(001)/H2O interface. J. Phys. Chem. C 118, 10980–10988 (2014).
Ketteler, G. et al. The nature of water nucleation sites on TiO2(110) surfaces revealed by ambient pressure X-ray photoelectron spectroscopy. J. Phys. Chem. C 111, 8278–8282 (2007).
Barker, A. S. Temperature dependence of the transverse and longitudinal optic mode frequencies and charges in SrTiO3 and BaTiO3 . Phys. Rev. 145, 391–399 (1966).
Fischer, B., Bäuerle, D. & Buckel, W. J. Surface polaritons in KTaO3 and SrTiO3 . Solid. State. Commun. 14, 291–294 (1974).
Wrighton, M. S. et al. Strontium titanate photoelectrodes. Efficient photoassisted electrolysis of water at zero applied potential. J. Am. Chem. Soc. 98, 2774–2779 (1976).
Zhu, Y., Uchida, H. & Watanabe, M. Oxidation of carbon monoxide at a platinum film electrode studied by Fourier transform infrared spectroscopy with attenuated total reflection technique. Langmuir 15, 8757–8764 (1999).
Zhu, Y., Uchida, H., Yajima, T. & Watanabe, M. Attenuated total reflection-Fourier transform infrared study of methanol oxidation on sputtered Pt film electrode. Langmuir 17, 146–154 (2001).
Fano, U. Effects of configuration interaction on intensities and phase shifts. Phys. Rev. 124, 1866–1878 (1961).
Gervais, F., Servoin, J., Baratoff, A., Bednorz, J. G. & Binnig, G. Temperature dependence of plasmons in Nb-doped SrTiO3 . Phys. Rev. B 47, 8187–8194 (1993).
Giguère, P. A. & Harvey, K. B. On the infrared absorption of water and heavy water in condensed states. Can. J. Chem. 34, 798–808 (1956).
Giustino, F. & Pasquarello, A. Infrared spectra at surfaces and interfaces from first principles: evolution of the spectra across the Si(100)–SiO2 interface. Phys. Rev. Lett. 95, 187402 (2005).
Milosevic, M. Internal Reflection and ATR Spectroscopy 264 (Wiley, 2012).
Acknowledgements
This research is based on work supported by the Air Force Office of Scientific Research (AFOSR, award no. FA9550-12-1-0337), which supplied the 64-element infrared array detector and partially supported a postdoctoral fellow, and by the Department of Energy (DOE) Office of Basic Energy Sciences (CPIMS program no. KC030102, FWP no. CH12CUK1), which supported two graduate students. The theory work of C.D.P. and D.P. was supported by a User Project at The Molecular Foundry (TMF), LBNL, with calculations performed on its computing resources, Nano and Vulcan, managed by the High Performance Computing Services Group of LBNL, and on the Cray XE6 Hopper computer at the National Energy Research Scientific Computing Center (NERSC), LBNL. Both TMF and NERSC are DOE Office of Science User Facilities supported by the Office of Science of the US Department of Energy (contract no. DE-AC02-05CH11231).
Author information
Authors and Affiliations
Contributions
T.C. conceived the project and wrote the manuscript with input from all authors. D.M.H. and M.M.W. constructed the transient set-up, collected transient data and prepared samples. X.C. collected and analysed the static reflectance measurements and characterized sample quantum efficiency. T.C. and D.M.H. analysed the transient data. C.D.P. and D.P. were responsible for the theoretical calculations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1905 kb)
Supplementary information
Supplementary Movie 1 (MPG 1435 kb)
Supplementary information
Supplementary Movie 2 (MPG 1373 kb)
Supplementary information
Supplementary Movie 3 (MPG 1291 kb)
Supplementary information
Supplementary Movie 4 (MPG 1280 kb)
Rights and permissions
About this article
Cite this article
Herlihy, D., Waegele, M., Chen, X. et al. Detecting the oxyl radical of photocatalytic water oxidation at an n-SrTiO3/aqueous interface through its subsurface vibration. Nature Chem 8, 549–555 (2016). https://doi.org/10.1038/nchem.2497
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2497
This article is cited by
-
Free energy difference to create the M-OH* intermediate of the oxygen evolution reaction by time-resolved optical spectroscopy
Nature Materials (2022)
-
The kinetics of metal oxide photoanodes from charge generation to catalysis
Nature Reviews Materials (2021)
-
Voltage- and time-dependent valence state transition in cobalt oxide catalysts during the oxygen evolution reaction
Nature Communications (2020)
-
Picosecond to millisecond tracking of a photocatalytic decarboxylation reaction provides direct mechanistic insights
Nature Communications (2019)
-
Selecting between two transition states by which water oxidation intermediates decay on an oxide surface
Nature Catalysis (2019)