Abstract
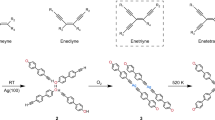
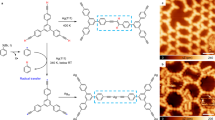
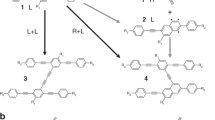
The Bergman cyclization is one of the most fascinating rearrangements in chemistry, with important implications in organic synthesis and pharmacology. Here we demonstrate a reversible Bergman cyclization for the first time. We induced the on-surface transformation of an individual aromatic diradical into a highly strained ten-membered diyne using atomic manipulation and verified the products by non-contact atomic force microscopy with atomic resolution. The diyne and diradical were stabilized by using an ultrathin NaCl film as the substrate, and the diyne could be transformed back into the diradical. Importantly, the diradical and the diyne exhibit different reactivity, electronic, magnetic and optical properties associated with the changes in the bond topology, and spin multiplicity. With this reversible, triggered Bergman cyclization we demonstrated switching on demand between the two reactive intermediates by means of selective C–C bond formation or cleavage, which opens up the field of radical chemistry for on-surface reactions by atomic manipulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jones, R. R. & Bergman, R. G. p-Benzyne. Generation as an intermediate in a thermal isomerization reaction and trapping evidence for the 1,4-benzenediyl structure. J. Am. Chem. Soc. 94, 660–661 (1972).
Wenk, H. H., Winkler, M. & Sander, W. One century of aryne chemistry. Angew. Chem. Int. Ed. 42, 502–528 (2003).
Nicolaou, K. C., Dai, W.-M., Tsay, S.-C., Estevez, V. A. & Wrasidlo, W. Designed enediynes: a new class of DNA-cleaving molecules with potent and selective anticancer activity. Science 256, 1172–1178 (1992).
Nicolaou, K., Smith, A. & Yue, E. Chemistry and biology of natural and designed enediynes. Proc. Natl Acad. Sci. USA 90, 5881–5888 (1993).
Sinha, S. C. et al. Prodrugs of dynemicin analogs for selective chemotherapy mediated by an aldolase catalytic Ab. Proc. Natl Acad. Sci. USA 101, 3095–3099 (2004).
Darby, N. et al. Concerning the 1,5-didehydro-[10]-annulene system. J. Chem. Soc. D 23, 1516–1517 (1971).
Chapman, O., Chang, C. & Kolc, J. 9,10-Dehydroanthracene. A derivative of 1,4-dehydrobenzene. J. Am. Chem. Soc. 98, 5703–5705 (1976).
Schottelius, M. J. & Chen, P. 9,10-Dehydroanthracene: p-benzyne-type biradicals abstract hydrogen unusually slowly. J. Am. Chem. Soc. 118, 4896–4903 (1996).
Wenk, H. H. & Sander, W. Photochemistry of 9,10-dicarbonyl-9,10-dihydroanthracene—a source of 9,10-dehydroanthracene? Eur. J. Org. Chem. 1999, 57–60 (1999).
Kötting, C., Sander, W., Kammermeier, S. & Herges, R. Matrix isolation of 3,4-benzocyclodeca-3,7,9-triene-1,5-diyne. Eur. J. Org. Chem. 1998, 799–803 (1998).
Perepichka, D. F. & Rosei, F. Extending polymer conjugation into the second dimension. Science 323, 216–217 (2009).
Palma, C.-A. & Samorì, P. Blueprinting macromolecular electronics. Nature Chem. 3, 431–436 (2011).
Hla, S.-W., Bartels, L., Meyer, G. & Rieder, K.-H. Inducing all steps of a chemical reaction with the scanning tunneling microscope tip: towards single molecule engineering. Phys. Rev. Lett. 85, 2777–2780 (2000).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nature Nanotech. 2, 687–691 (2007).
Cai, J. et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466, 470–473 (2010).
Lafferentz, L. et al. Controlling on-surface polymerization by hierarchical and substrate-directed growth. Nature Chem. 4, 215–220 (2012).
Sun, Q. et al. On-surface formation of one-dimensional polyphenylene through Bergman cyclization. J. Am. Chem. Soc. 135, 8448–8451 (2013).
de Oteyza, D. G. et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 340, 1434–1437 (2013).
Stipe, B. et al. Single-molecule dissociation by tunneling electrons. Phys. Rev. Lett. 78, 4410–4413 (1997).
Lee, H. J. & Ho, W. Single-bond formation and characterization with a scanning tunneling microscope. Science 286, 1719–1722 (1999).
Zhao, A. et al. Controlling the Kondo effect of an adsorbed magnetic ion through its chemical bonding. Science 309, 1542–1544 (2005).
Repp, J., Meyer, G., Paavilainen, S., Olsson, F. E. & Persson, M. Imaging bond formation between a gold atom and pentacene on an insulating surface. Science 312, 1196–1199 (2006).
Liljeroth, P., Repp, J. & Meyer, G. Current-induced hydrogen tautomerization and conductance switching of naphthalocyanine molecules. Science 317, 1203–1206 (2007).
Albrecht, F., Neu, M., Quest, C., Swart, I. & Repp, J. Formation and characterization of a molecule–metal–molecule bridge in real space. J. Am. Chem. Soc. 135, 9200–9203 (2013).
Kumagai, T. et al. Controlling intramolecular hydrogen transfer in a porphycene molecule with single atoms or molecules located nearby. Nature Chem. 6, 41–46 (2014).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Mohn, F. et al. Reversible bond formation in a gold-atom–organic-molecule complex as a molecular switch. Phys. Rev. Lett. 105, 266102 (2010).
Pavliček, N. et al. On-surface generation and imaging of arynes by atomic force microscopy. Nature Chem. 7, 623–628 (2015).
Riss, A. et al. Local electronic and chemical structure of oligo-acetylene derivatives formed through radical cyclizations at a surface. Nano Lett. 14, 2251–2255 (2014).
Pavliček, N. et al. Atomic force microscopy reveals bistable configurations of dibenzo[a,h]thianthrene and their interconversion pathway. Phys. Rev. Lett. 108, 086101 (2012).
Schuler, B. et al. Adsorption geometry determination of single molecules by atomic force microscopy. Phys. Rev. Lett. 111, 106103 (2013).
Mohn, F., Schuler, B., Gross, L. & Meyer, G. Different tips for high-resolution AFM and STM imaging of single molecules. Appl. Phys. Lett. 102, 073109 (2013).
Repp, G., Meyer, G., Stojkovic, S. M., Gourdon, A. & Joachim, C. Molecules on insulating films: scanning-tunneling microscopy imaging of individual molecular orbitals. Phys. Rev. Lett. 94, 026803 (2005).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Moll, N. et al. Image distortions of partly fluorinated hydrocarbons in atomic force microscopy with carbon monoxide terminated tips. Nano Lett. 14, 6127–6131 (2014).
Swart, I., Sonnleitner, T., Niedenführ, J. & Repp, J. Controlled lateral manipulation of molecules on insulating films by STM. Nano Lett. 12, 1070–1074 (2012).
Gross, L. et al. Organic structure determination using atomic resolution scanning probe microscopy. Nature Chem. 2, 821–825 (2010).
Giessibl, F. J. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 73, 3956–3958 (1998).
Albrecht, T. R., Grütter, P., Horne, D. & Rugar, D. Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J. Appl. Phys. 69, 668–673 (1991).
Boneschanscher, M. P., Hämäläinen, S. K., Liljeroth, P. & Swart, I. Sample corrugation affects the apparent bond lengths in atomic force microscopy. ACS Nano 8, 3006–3014 (2014).
Hapala, P. et al. The mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964).
Blum, V. et al. Ab initio molecular simulations with numeric atom-centered orbitals. Comp. Phys. Comm. 180, 2175–2196 (2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Tkatchenko, A. & Scheffler, M. Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 102, 073005 (2009).
Ruiz, V. G., Liu, W., Zojer, E., Scheffler, M. & Tkatchenko, A. Density-functional theory with screened van-der-Waals interactions for the modeling of hybrid inorganic–organic systems. Phys. Rev. Lett. 108, 146103 (2012).
Zhang, G.-X., Tkatchenko, A., Paier, J., Appel, H. & Scheffler, M. Van der Waals interactions in ionic and semiconductor solids. Phys. Rev. Lett. 107, 245501 (2011).
Acknowledgements
We thank I. Tavernelli, D. Pérez, E. Guitián and R. Allenspach for discussions. We acknowledge financial support from the European Research Council Advanced Grant CEMAS (agreement no. 291194), the European Union project PAMS (610446) and the Initial Training Network's QTea (317485) and ACRITAS (317348) programs. D.P. acknowledges the Spanish Ministry of Science and Competitiveness for financial support (MAT2013-46593-C6-6-P).
Author information
Authors and Affiliations
Contributions
B.S., S.F., F.M., N.P., G.M. and L.G. performed the STM/AFM experiments. N.M. performed the DFT calculations. D.P. identified the reaction. All the authors analysed the data and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 586 kb)
Rights and permissions
About this article
Cite this article
Schuler, B., Fatayer, S., Mohn, F. et al. Reversible Bergman cyclization by atomic manipulation. Nature Chem 8, 220–224 (2016). https://doi.org/10.1038/nchem.2438
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2438
This article is cited by
-
Universal inter-molecular radical transfer reactions on metal surfaces
Nature Communications (2024)
-
Organic radicals in single-molecule junctions
Science China Materials (2024)
-
On-surface synthesis of aromatic cyclo[10]carbon and cyclo[14]carbon
Nature (2023)
-
Local probe-induced structural isomerization in a one-dimensional molecular array
Nature Communications (2023)
-
3 + 3 makes the ring
Nature Synthesis (2022)