Abstract
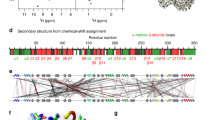
Biomacromolecules that are challenging for the usual structural techniques can be studied with atomic resolution by solid-state NMR spectroscopy. However, the paucity of distance restraints >5 Å, traditionally derived from measurements of magnetic dipole–dipole couplings between protein nuclei, is a major bottleneck that hampers such structure elucidation efforts. Here, we describe a general approach that enables the rapid determination of global protein fold in the solid phase via measurements of nuclear paramagnetic relaxation enhancements (PREs) in several analogues of the protein of interest containing covalently attached paramagnetic tags, without the use of conventional internuclear distance restraints. The method is demonstrated using six cysteine–EDTA–Cu2+ mutants of the 56-residue B1 immunoglobulin-binding domain of protein G, for which ~230 longitudinal backbone 15N PREs corresponding to distances of ~10–20 Å were obtained. The mean protein fold determined in this manner agrees with the X-ray structure with a backbone atom root-mean-square deviation of 1.8 Å.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wüthrich, K. NMR studies of structure and function of biological macromolecules (Nobel lecture). Angew. Chem. Int. Ed. 42, 3340–3363 (2003).
Lange, A. et al. Toxin-induced conformational changes in a potassium channel revealed by solid-state NMR. Nature 440, 959–962 (2006).
Cady, S. D. et al. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature 463, 689–692 (2010).
Petkova, A. T. et al. Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science 307, 262–265 (2005).
Wasmer, C. et al. Amyloid fibrils of the HET-s(218–289) prion form a β solenoid with a triangular hydrophobic core. Science 319, 1523–1526 (2008).
Castellani, F. et al. Structure of a protein determined by solid-state magic-angle spinning NMR spectroscopy. Nature 420, 98–102 (2002).
Zech, S. G., Wand, A. J. & McDermott, A. E. Protein structure determination by high-resolution solid-state NMR spectroscopy: application to microcrystalline ubiquitin. J. Am. Chem. Soc. 127, 8618–8626 (2005).
Loquet, A. et al. 3D structure determination of the Crh protein from highly ambiguous solid-state NMR restraints. J. Am. Chem. Soc. 130, 3579–3589 (2008).
Manolikas, T., Herrmann, T. & Meier, B. H. Protein structure determination from 13C spin-diffusion solid-state NMR spectroscopy. J. Am. Chem. Soc. 130, 3959–3966 (2008).
Korukottu, J. et al. High-resolution 3D structure determination of kaliotoxin by solid-state NMR spectroscopy. PLoS ONE 3, e2359 (2008).
Franks, W. T. et al. Dipole tensor-based atomic-resolution structure determination of a nanocrystalline protein by solid-state NMR. Proc. Natl Acad. Sci. USA 105, 4621–4626 (2008).
De Paëpe, G., Lewandowski, J. R., Loquet, A., Böckmann, A. & Griffin, R. G. Proton assisted recoupling and protein structure determination. J. Chem. Phys. 129, 245101 (2008).
Robustelli, P., Cavalli, A. & Vendruscolo, M. Determination of protein structures in the solid state from NMR chemical shifts. Structure 16, 1764–1769 (2008).
Shen, Y., Vernon, R., Baker, D. & Bax, A. De novo protein structure generation from incomplete chemical shift assignments. J. Biomol. NMR 43, 63–78 (2009).
Nieuwkoop, A. J., Wylie, B. J., Franks, W. T., Shah, G. J. & Rienstra, C. M. Atomic resolution protein structure determination by three-dimensional transferred echo double resonance solid-state nuclear magnetic resonance spectroscopy. J. Chem. Phys. 131, 095101 (2009).
Zhang, Y. et al. Resonance assignment and three-dimensional structure determination of a human alpha-defensin, HNP-1, by solid-state NMR. J. Mol. Biol. 397, 408–422 (2010).
Jehle, S. et al. Solid-state NMR and SAXS studies provide a structural basis for the activation of αB-crystallin oligomers. Nature Struct. Mol. Biol. 17, 1037–1042 (2010).
Linser, R., Bardiaux, B., Higman, V., Fink, U. & Reif, B. Structure calculation from unambiguous long-range amide and methyl 1H–1H distance restraints for a microcrystalline protein with MAS solid-state NMR spectroscopy. J. Am. Chem. Soc. 133, 5905–5912 (2011).
Huber, M. et al. A proton-detected 4D solid-state NMR experiment for protein structure determination. ChemPhysChem 12, 915–918 (2011).
Helmus, J. J., Nadaud, P. S., Höfer, N. & Jaroniec, C. P. Determination of methyl 13C–15N dipolar couplings in peptides and proteins by three-dimensional and four-dimensional magic-angle spinning solid-state NMR spectroscopy. J. Chem. Phys. 128, 052314 (2008).
Jaroniec, C. P., Filip, C. & Griffin, R. G. 3D TEDOR NMR experiments for the simultaneous measurement of multiple carbon–nitrogen distances in uniformly 13C,15N-labeled solids. J. Am. Chem. Soc. 124, 10728–10742 (2002).
Takegoshi, K., Nakamura, S. & Terao, T. 13C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem. Phys. Lett. 344, 631–637 (2001).
Lange, A., Luca, S. & Baldus, M. Structural constraints from proton-mediated rare-spin correlation spectroscopy in rotating solids. J. Am. Chem. Soc. 124, 9704–9705 (2002).
Solomon, I. Relaxation processes in a system of two spins. Phys. Rev. 99, 559–565 (1955).
Bertini, I., Luchinat, C. & Parigi, G. Solution NMR of Paramagnetic Molecules: Applications to Metallobiomolecules and Models (Elsevier, 2001).
Gillespie, J. R. & Shortle, D. Characterization of long-range structure in the denatured state of staphylococcal nuclease. II. Distance restraints from paramagnetic relaxation and calculation of an ensemble of structures. J. Mol. Biol. 268, 170–184 (1997).
Battiste, J. L. & Wagner, G. Utilization of site-directed spin labeling and high-resolution heteronuclear nuclear magnetic resonance for global fold determination of large proteins with limited nuclear overhauser effect data. Biochemistry 39, 5355–5365 (2000).
Gaponenko, V. et al. Protein global fold determination using site-directed spin and isotope labeling. Protein Sci. 9, 302–309 (2000).
Pintacuda, G. et al. Solid-state NMR spectroscopy of a paramagnetic protein: assignment and study of human dimeric oxidized CuII–ZnII superoxide dismutase (SOD). Angew. Chem. Int. Ed. 46, 1079–1082 (2007).
Balayssac, S., Bertini, I., Lelli, M., Luchinat, C. & Maletta, M. Paramagnetic ions provide structural restraints in solid-state NMR of proteins. J. Am. Chem. Soc. 129, 2218–2219 (2007).
Balayssac, S., Bertini, I., Bhaumik, A., Lelli, M. & Luchinat, C. Paramagnetic shifts in solid-state NMR of proteins to elicit structural information. Proc. Natl Acad. Sci. USA 105, 17284–17289 (2008).
Bertini, I. et al. High-resolution solid-state NMR structure of a 17.6 kDa protein. J. Am. Chem. Soc. 132, 1032–1040 (2010).
Nadaud, P. S., Helmus, J. J., Höfer, N. & Jaroniec, C. P. Long-range structural restraints in spin-labeled proteins probed by solid-state nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 129, 7502–7503 (2007).
Nadaud, P. S., Helmus, J. J., Kall, S. L. & Jaroniec, C. P. Paramagnetic ions enable tuning of nuclear relaxation rates and provide long-range structural restraints in solid-state NMR of proteins. J. Am. Chem. Soc. 131, 8108–8120 (2009).
Nadaud, P. S., Helmus, J. J., Sengupta, I. & Jaroniec, C. P. Rapid acquisition of multidimensional solid-state NMR spectra of proteins facilitated by covalently bound paramagnetic tags. J. Am. Chem. Soc. 132, 9561–9563 (2010).
Nadaud, P. S., Sengupta, I., Helmus, J. J. & Jaroniec, C. P. Evaluation of the influence of intermolecular electron–nucleus couplings and intrinsic metal binding sites on the measurement of 15N longitudinal paramagnetic relaxation enhancements in proteins by solid-state NMR. J. Biomol. NMR 51, 293–302 (2011).
Ermácora, M. R., Delfino, J. M., Cuenoud, B., Schepartz, A. & Fox, R. O. Conformation-dependent cleavage of staphylococcal nuclease with a disulfide-linked iron chelate. Proc. Natl Acad. Sci. USA 89, 6383–6387 (1992).
Hubbell, W. L. & Altenbach, C. Investigation of structure and dynamics in membrane proteins using site-directed spin labeling. Curr. Opin. Struct. Biol. 4, 566–573 (1994).
Schwieters, C. D., Kuszewski, J. J., Tjandra, N. & Clore, G. M. The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 160, 65–73 (2003).
Gallagher, T., Alexander, P., Bryan, P. & Gilliland, G. L. Two crystal structures of the B1 immunoglobulin-binding domain of streptococcal protein G and comparison with NMR. Biochemistry 33, 4721–4729 (1994).
Franks, W. T., Wylie, B. J., Stellfox, S. A. & Rienstra, C. M. Backbone conformational constraints in a microcrystalline U–15N-labeled protein by 3D dipolar-shift solid-state NMR spectroscopy. J. Am. Chem. Soc. 128, 3154–3155 (2006).
Shen, Y., Delaglio, F., Cornilescu, G. & Bax, A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 44, 213–223 (2009).
Rost, B. & Sander, C. Conservation and prediction of solvent accessibility in protein families. Proteins 20, 216–226 (1994).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Iwahara, J., Schwieters, C. D. & Clore, G. M. Ensemble approach for NMR structure refinement against 1H paramagnetic relaxation enhancement data arising from a flexible paramagnetic group attached to a macromolecule. J. Am. Chem. Soc. 126, 5879–5896 (2004).
Kuszewski, J., Gronenborn, A. M. & Clore, G. M. Improving the packing and accuracy of NMR structures with a pseudopotential for the radius of gyration. J. Am. Chem. Soc. 121, 2337–2338 (1999).
Kuszewski, J., Gronenborn, A. M. & Clore, G. M. Improving the quality of NMR and crystallographic protein structures by means of a conformational database potential derived from structure databases. Protein Sci. 5, 1067–1080 (1996).
Grishaev, A. & Bax, A. An empirical backbone–backbone hydrogen-bonding potential in proteins and its applications to NMR structure refinement and validation. J. Am. Chem. Soc. 126, 7281–7292 (2004).
Ryabov, Y., Suh, J-Y., Grishaev, A., Clore, G. M. & Schwieters, C. D. Using the experimentally determined components of the overall rotational diffusion tensor to restrain molecular shape and size in NMR structure determination of globular proteins and protein–protein complexes. J. Am. Chem. Soc. 131, 9522–9531 (2009).
Schwieters, C. D. & Clore, G. M. Reweighted atomic densities to represent ensembles of NMR structures. J. Biomol. NMR 23, 221–225 (2002).
Acknowledgements
This research was supported by the National Science Foundation (CAREER award MCB-0745754 to C.P.J.). C.D.S. was supported by the National Institutes of Health Intramural Research Program of the Center for Information Technology. The GB1 plasmid was kindly provided by A.M. Gronenborn.
Author information
Authors and Affiliations
Contributions
C.P.J. designed the research. I.S. and P.S.N. prepared the samples. I.S., P.S.N. and J.J.H. recorded and analysed the NMR data. C.D.S. and C.P.J. performed the structure calculations. C.D.S. and C.P.J. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 371 kb)
Rights and permissions
About this article
Cite this article
Sengupta, I., Nadaud, P., Helmus, J. et al. Protein fold determined by paramagnetic magic-angle spinning solid-state NMR spectroscopy. Nature Chem 4, 410–417 (2012). https://doi.org/10.1038/nchem.1299
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1299
This article is cited by
-
Structure of membrane diacylglycerol kinase in lipid bilayers
Communications Biology (2021)
-
Rotamer Modelling of Cu(II) Spin Labels Based on the Double-Histidine Motif
Applied Magnetic Resonance (2018)
-
Dynamic intramolecular regulation of the histone chaperone nucleoplasmin controls histone binding and release
Nature Communications (2017)
-
Gd3+-chelated lipid accelerates solid-state NMR spectroscopy of seven-transmembrane proteins
Journal of Biomolecular NMR (2017)
-
A quantum spin-probe molecular microscope
Nature Communications (2016)