Abstract
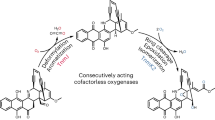
Elucidation of natural product biosynthetic pathways provides important insights into the assembly of potent bioactive molecules, and expands access to unique enzymes able to selectively modify complex substrates. Here, we show full reconstitution, in vitro, of an unusual multi-step oxidative cascade for post-assembly-line tailoring of tirandamycin antibiotics. This pathway involves a remarkably versatile and iterative cytochrome P450 monooxygenase (TamI) and a flavin adenine dinucleotide-dependent oxidase (TamL), which act co-dependently through the repeated exchange of substrates. TamI hydroxylates tirandamycin C (TirC) to generate tirandamycin E (TirE), a previously unidentified tirandamycin intermediate. TirE is subsequently oxidized by TamL, giving rise to the ketone of tirandamycin D (TirD), after which a unique exchange back to TamI enables successive epoxidation and hydroxylation to afford, respectively, the final products tirandamycin A (TirA) and tirandamycin B (TirB). Ligand-free, substrate- and product-bound crystal structures of bicovalently flavinylated TamL oxidase reveal a likely mechanism for the C10 oxidation of TirE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carlson, J. C., Li, S., Burr, D. A. & Sherman, D. H. Isolation and characterization of tirandamycins from a marine-derived Streptomyces sp. J. Nat. Prod. 72, 2076–2079 (2009).
Royles, B. J. L. Naturally occurring tetramic acids — structure, isolation, and synthesis. Chem. Rev. 95, 1981–2001 (1995).
Carlson, J. C. et al. Identification of the tirandamycin biosynthetic gene cluster from Streptomyces sp. 307-9. ChemBioChem 11, 564–572 (2010).
Omura, T. & Sato, R. The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J. Biol. Chem. 239, 2379–2385 (1964).
Li, S., Podust, L. M. & Sherman, D. H. In vitro characterization of a self-sufficient biosynthetic cytochrome P450 PikC fused to a heterologous reductase domain RhFRED. J. Am. Chem. Soc. 129, 12940–12941 (2007).
Dickens, M. L., Priestley, N. D. & Strohl, W. R. In vivo and in vitro bioconversion of epsilon-rhodomycinone glycoside to doxorubicin: functions of DauP, DauK, and DoxA. J. Bacteriol. 179, 2641–2650 (1997).
Ro, D. K. et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943 (2006).
Kutchan, T. M. & Dittrich, H. Characterization and mechanism of the berberine bridge enzyme, a covalently flavinylated oxidase of benzophenanthridine alkaloid biosynthesis in plants. J. Biol. Chem. 270, 24475–24481 (1995).
Winkler, A. et al. A concerted mechanism for berberine bridge enzyme. Nat. Chem. Biol. 4, 739–741 (2008).
Huang, C. H. et al. Crystal structure of glucooligosaccharide oxidase from Acremonium strictum: a novel flavinylation of 6-S-cysteinyl, 8alpha-N1-histidyl FAD. J. Biol. Chem. 280, 38831–38838 (2005).
Alexeev, I., Sultana, A., Mantsala, P., Niemi, J. & Schneider, G. Aclacinomycin oxidoreductase (AknOx) from the biosynthetic pathway of the antibiotic aclacinomycin is an unusual flavoenzyme with a dual active site. Proc. Natl Acad. Sci. USA 104, 6170–6175 (2007).
Sosio, M., Stinchi, S., Beltrametti, F., Lazzarini, A. & Donadio, S. The gene cluster for the biosynthesis of the glycopeptide antibiotic A40926 by Nonomuraea species. Chem. Biol. 10, 541–549 (2003).
Rand, T., Qvist, K. B., Walter, C. P. & Poulsen, C. H. Characterization of the flavin association in hexose oxidase from Chondrus crispus. FEBS J. 273, 2693–2703 (2006).
Winkler, A. et al. Structural and mechanistic studies reveal the functional role of bicovalent flavinylation in berberine bridge enzyme. J. Biol. Chem. 284, 19993–20001 (2009).
Winkler, A. et al. Berberine bridge enzyme catalyzes the six electron oxidation of (S)-reticuline to dehydroscoulerine. Phytochemistry 70, 1092–1097 (2009).
Mathews, F. S., Chen, Z. W., Bellamy, H. D. & McIntire, W. S. Three-dimensional structure of p-cresol methylhydroxylase (flavocytochrome c) from Pseudomonas putida at 3.0-Å resolution. Biochemistry 30, 238–247 (1991).
Carter, C. J. & Thornburg, R. W. Tobacco nectarin V is a flavin-containing berberine bridge enzyme-like protein with glucose oxidase activity. Plant Physiol. 134, 460–469 (2004).
Willie, A., Edmondson, D. E. & Jorns, M. S. Sarcosine oxidase contains a novel covalently bound FMN. Biochemistry 35, 5292–5299 (1996).
Fitzpatrick, P. F. Carbanion versus hydride transfer mechanisms in flavoprotein-catalyzed dehydrogenations. Bioorg. Chem. 32, 125–139 (2004).
Fraaije, M. W. & Mattevi, A. Flavoenzymes: diverse catalysts with recurrent features. Trends Biochem. Sci. 25, 126–132 (2000).
Liu, Y. C. et al. Interception of teicoplanin oxidation intermediates yields new antimicrobial scaffolds. Nat. Chem. Biol. 7, 304–309 (2011).
Ghisla, S. & Massey, V. Mechanisms of flavoprotein-catalyzed reactions. Eur. J. Biochem. 181, 1–17 (1989).
Zhang, Z. et al. Structural origins of the selectivity of the trifunctional oxygenase clavaminic acid synthase. Nat. Struct. Biol. 7, 127–133 (2000).
Salowe, S. P., Marsh, E. N. & Townsend, C. A. Purification and characterization of clavaminate synthase from Streptomyces clavuligerus: an unusual oxidative enzyme in natural product biosynthesis. Biochemistry 29, 6499–6508 (1990).
Sherman, D. H. et al. The structural basis for substrate anchoring, active site selectivity, and product formation by P450 PikC from Streptomyces venezuelae. J. Biol. Chem. 281, 26289–26297 (2006).
Tokai, T. et al. Fusarium Tri4 encodes a key multifunctional cytochrome P450 monooxygenase for four consecutive oxygenation steps in trichothecene biosynthesis. Biochem. Biophys. Res. Commun. 353, 412–417 (2007).
Anzai, Y. et al. Functional analysis of MycCI and MycG, cytochrome P450 enzymes involved in biosynthesis of mycinamicin macrolide antibiotics. Chem. Biol. 15, 950–959 (2008).
Huang, C. H. et al. Functional roles of the 6-S-cysteinyl, 8alpha-N1-histidyl FAD in glucooligosaccharide oxidase from Acremonium strictum. J. Biol. Chem. 283, 30990–30996 (2008).
Winkler, A., Kutchan, T. M. & Macheroux, P. 6-S-cysteinylation of bi-covalently attached FAD in berberine bridge enzyme tunes the redox potential for optimal activity. J. Biol. Chem. 282, 24437–24443 (2007).
Holton, J. & Alber, T. Automated protein crystal structure determination using ELVES. Proc. Natl Acad. Sci. USA 101, 1537–1542 (2004).
Collaborative Computational Project, Number 4. Acta Crystallogr. D 50, 760–763 (1994).
Acknowledgements
The authors thank P. G. Cruz for assistance with NMR structure elucidation, P. M. Kells for excellent technical assistance, and staff members of Beamline 8.3.1, J. Holton, G. Meigs and J. Tanamachi at the Advanced Light Source at Lawrence Berkeley National Laboratory, for assistance with data collection. This work was funded by the National Institutes of Health (grant GM078553 to D.H.S. and L.M.P.), International Cooperative Biodiversity Group (U01 TW007404) and the Hans W. Vahlteich Professorship (to D.H.S.). The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy (contract no. DE-AC02-05CH11231).
Author information
Authors and Affiliations
Contributions
All authors designed experiments and analysed data. J.C., S.L., S.G., Y.A. and D.B. performed the experiments. J.C., S.L., L.P. and D.S. wrote the manuscript. J.C. and S.L. contributed equally to this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1655 kb)
Rights and permissions
About this article
Cite this article
Carlson, J., Li, S., Gunatilleke, S. et al. Tirandamycin biosynthesis is mediated by co-dependent oxidative enzymes. Nature Chem 3, 628–633 (2011). https://doi.org/10.1038/nchem.1087
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1087
This article is cited by
-
Endowing homodimeric carbamoyltransferase GdmN with iterative functions through structural characterization and mechanistic studies
Nature Communications (2022)
-
Discovery and characterization of a terpene biosynthetic pathway featuring a norbornene-forming Diels-Alderase
Nature Communications (2022)
-
An overview of the cytochrome P450 enzymes that catalyze the same-site multistep oxidation reactions in biotechnologically relevant selected actinomycete strains
Applied Microbiology and Biotechnology (2021)
-
Cytotoxic anthracycline and antibacterial tirandamycin analogues from a marine-derived Streptomyces sp. SCSIO 41399
The Journal of Antibiotics (2019)
-
Cytochrome P450 oxidase SlgO1 catalyzes the biotransformation of tirandamycin C to a new tirandamycin derivative
3 Biotech (2019)