Abstract
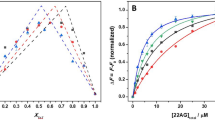
Ligands that stabilize the formation of telomeric DNA G-quadruplexes have potential as cancer treatments, because the G-quadruplex structure cannot be extended by telomerase, an enzyme over-expressed in many cancer cells. Understanding the kinetic, thermodynamic and mechanical properties of small-molecule binding to these structures is therefore important, but classical ensemble assays are unable to measure these simultaneously. Here, we have used a laser tweezers method to investigate such interactions. With a force jump approach, we observe that pyridostatin promotes the folding of telomeric G-quadruplexes. The increased mechanical stability of pyridostatin-bound G-quadruplex permits the determination of a dissociation constant Kd of 490 ± 80 nM. The free-energy change of binding obtained from a Hess-like process provides an identical Kd for pyridostatin and a Kd of 42 ± 3 µM for a weaker ligand RR110. We anticipate that this single-molecule platform can provide detailed insights into the mechanical, kinetic and thermodynamic properties of liganded bio-macromolecules, which have biological relevance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gellert, M., Lipsett, M. N. & Davies, D. R. Helix formation by guanylic acid. Proc. Natl Acad. Sci. USA 48, 2013–2018 (1962).
Gilbert, D. E. & Feigon, J. Multistranded DNA structures. Curr. Opin. Struct. Biol. 9, 305–314 (1999).
Sundquist, W. I. & Heaphy, S. Evidence for interstrand quadruplex formation in the dimerization of human immunodeficiency virus 1 genomic RNA. Proc. Natl Acad. Sci. USA 90, 3393–3397 (1993).
Henderson, E., Hardin, C. C., Walk, S. K., Tinoco, I. Jr & Blackburn, E. H. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine·guanine base pairs. Cell 51, 899–908 (1987).
Moyzis, R. K. et al. A highly conservedrepetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl Acad. Sci. USA 85, 6622–6626 (1988).
Zhang, X., Mar, V., Zhou, W., Harrington, L. & Robinson, M. O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 13, 2388–2399 (1999).
Rezler, E. M., Bearss, D. J. & Hurley, L. H. Telomere inhibition and telomere disruption as processes for drug targeting. Annu. Rev. Pharmacol. Toxicol. 43, 359–379 (2003).
Kim, N. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 (1994).
Shin-ya, K. et al. Telomestatin, a novel telomerase inhibitor from Streptomyces anulatus. J. Am. Chem. Soc. 123, 1262–1263 (2001).
Kim, M.-Y., Vankayalapati, H., Shin-ya, K., Wierzba, K. a. & Hurley, L. H. Telomestatin, a potent telomerase inhibitor that interacts quite specifically with the human telomeric intramolecular G-quadruplex. J. Am. Chem. Soc. 124, 2098–2099 (2002).
Kerwin, S. M. G-quadruplex DNA as a target for drug design. Curr. Pharm. Des. 6, 441–478 (2000).
Patel, D. J., Phan, A. T. & Kuryavyi, V. Human telomere, oncogenic promoter and 5′-UTR G-quadruplexes: diverse higher order DNA and RNA targets for cancer therapeutics. Nucleic Acids Res. 35, 7429–7455 (2007).
Balasubramanian, S. & Neidle, S. G-quadruplex nucleic acids as therapeutic targets. Curr. Opin. Chem. Biol. 13, 345–353 (2009).
Greider, C. W. & Blackburn, E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337, 331–337 (1989).
Yu, Z. et al. ILPR G-quadruplexes formed in seconds demonstrate high mechanical stabilities. J. Am. Chem. Soc. 131, 1876–1882 (2009).
Galburt, E. A. et al. Backtracking determines the force sensitivity of RNAP II in a factor-dependent manner. Nature 446, 820–823 (2007).
Mejia, Y. X., Mao, H., Forde, N. R. & Bustamante, C. Thermal probing of E. coli RNA polymerase off-pathway mechanisms. J. Mol. Biol. 382, 628–637 (2008).
Parkinson, G. N., Lee, M. P. H. & Neidle, S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature 417, 876–880 (2002).
Luu, K. N., Phan, A. T., Kuryavyi, V., Lacroix, L. & Patel, D. J. Structure of the human telomere in K+ solution: an intramolecular (3 + 1) G-quadruplex scaffold. J. Am. Chem. Soc. 128, 9963–9970 (2006).
Ambrus, A. et al. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Res. 34, 2723–2735 (2006).
Mergny, J.-L., De Cian, A., Ghelab, A., Saccà, B. & Lacroix, L. Kinetics of tetramolecular quadruplexes. Nucleic Acids Res. 33, 81–94 (2005).
Zhao, Y. et al. Determining the folding and unfolding rate constants of nucleic acids by biosensor. Application to telomere G-quadruplex. J. Am. Chem. Soc. 126, 13255–13264 (2004).
Ying, L., Green, J. J., Li, H., Klenerman, D. & Balasubramanian, S. Studies on the structure and dynamics of the human telomeric G quadruplex by single-molecule fluorescence resonance energy transfer. Proc. Natl Acad. Sci. USA 100, 14629–14634 (2003).
Lee, J. Y., Okumus, B., Kim, D. S. & Ha, T. Extreme conformational diversity in human telomeric DNA. Proc. Natl Acad. Sci. USA 102, 18938–18943 (2005).
Jena, P. V. et al. G-quadruplex DNA bound by a synthetic ligand is highly dynamic. J. Am. Chem. Soc. 131, 12522–12523 (2009).
Rodriguez, R. et al. A novel small molecule that alters shelterin integrity and triggers a DNA-damage response at telomeres. J. Am. Chem. Soc. 130, 15758–15759 (2008).
Müller, S., Kumari, S., Rodriguez, R. & Balasubramanian, S. Small-molecule-mediated G-quadruplex isolation from human cells. Nature Chem. 2, 1095–1098 (2010).
Bugaut, A., Rodriguez, R., Kumari, S., Hsu, S-T. D. & Balasubramanian, S. Small molecule-mediated inhibition of translation by targeting a native RNA G-quadruplex. Org. Biomol. Chem. 8, 2771–2776 (2010).
Silberberg, M. S. Chemistry: The Molecular Nature of Matter and Change 5th edn (McGraw-Hill, 2009).
Jarzynski, C. Nonequilibrium equality for free energy differences. Phys. Rev. Lett. 78, 2690–2693 (1997).
Liphardt, J., Dumont, S., Smith, S. B., Tinoco, I. Jr & Bustamante, C. Equilibrium information from nonequilibrium measurements in an experimental test of Jarzynski's equality. Science 296, 1832–1835 (2002).
Balagurumoorthy, P. & Brahmachari, S. K. Structure and stability of human telomeric sequence. J. Biol. Chem. 269, 21858–21869 (1994).
Li, W., Wu, P. Ohmichi, T. & Sugimoto, N. Characterization and thermodynamic properties of quadruplex/duplex competition. FEBS Lett. 526, 77–81 (2002).
Lane, A. N., Chaires, J. B., Gray, R. D. & Trent, J. O. Stability and kinetics of G-quadruplex structures. Nucleic Acids Res. 36, 5482–5515 (2008).
Tran, P. L. T., Mergny, J-L. & Alberti, P. Stability of telomeric G-quadruplexes. Nucleic Acids Res. 39, 3282–3294 (2011).
Dhakal, S. et al. Coexistence of an ILPR i-motif and a partially folded structure with comparable mechanical stability revealed at the single-molecule level. J. Am. Chem. Soc. 132, 8991–8997 (2010).
Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918).
Mao, H. & Luchette, P. An integrated laser tweezers instrument for microanalysis of individual protein aggregates. Sens. Actuat. B 129, 764–771 (2008).
Baumann, C. G., Smith, S. B., Bloomfield, V. A. & Bustamante, C. Ionic effects on the elasticity of single DNA molecules. Proc. Natl Acad. Sci. USA 94, 6185–6190 (1997).
Greenleaf, W. J., Frieda, K. L., Foster, D. A. N., Woodside, M. T. & Block, S. M. Direct observation of hierarchical folding in single riboswitch aptamers. Science 319, 630–633 (2008).
Acknowledgements
The authors thank the New Faculty Award Program at the Camille and Henry Dreyfus Foundation, Ohio Board of Regents, NSF CHE-1026532, and NIH R15 DK081191-01 for support to H.M., the BBSRC (UK) for a studentship to B.A., and Cancer Research UK for programme funding to S.B. This work was also supported by the Core Research for Evolutional Science and Technology (CREST) of JST and a Grant-in-Aid for Science Research from MEXT (Japan) to H.S.
Author information
Authors and Affiliations
Contributions
H.M., S.B. and H.S. formulated the concept for the project. H.M. and D.K. designed the experiments. D.K. performed the experiments and analysed the data. S.D. contributed to the kinetics experiment. B.A. and R.R. synthesized the ligands. Y.S. prepared the DNA construct. H.M., S.B. and H.S. wrote the manuscript with D.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 512 kb)
Rights and permissions
About this article
Cite this article
Koirala, D., Dhakal, S., Ashbridge, B. et al. A single-molecule platform for investigation of interactions between G-quadruplexes and small-molecule ligands. Nature Chem 3, 782–787 (2011). https://doi.org/10.1038/nchem.1126
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1126
This article is cited by
-
Chemo-mechanical forces modulate the topology dynamics of mesoscale DNA assemblies
Nature Communications (2023)
-
The ALS/FTD-related C9orf72 hexanucleotide repeat expansion forms RNA condensates through multimolecular G-quadruplexes
Nature Communications (2023)
-
Dynamic interaction of BRCA2 with telomeric G-quadruplexes underlies telomere replication homeostasis
Nature Communications (2022)
-
Visualising G-quadruplex DNA dynamics in live cells by fluorescence lifetime imaging microscopy
Nature Communications (2021)
-
Single-molecule imaging reveals replication fork coupled formation of G-quadruplex structures hinders local replication stress signaling
Nature Communications (2021)