Abstract
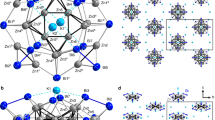
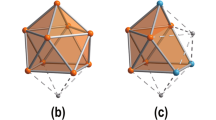
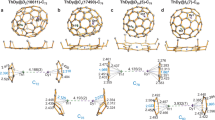
Heteromultimetallic hydride clusters containing both rare-earth and d-transition metals are of interest in terms of both their structure and reactivity. However, such heterometallic complexes have not yet been investigated to a great extent because of difficulties in their synthesis and structural characterization. Here, we report the synthesis, X-ray and neutron diffraction studies, and hydrogen addition and release properties of a family of rare-earth/d-transition-metal heteromultimetallic polyhydride complexes of the core structure type ‘Ln4MHn’ (Ln = Y, Dy, Ho; M = Mo, W; n = 9, 11, 13). Monitoring of hydrogen addition to a hydride cluster such as [{(C5Me4SiMe3)Y}4(μ-H)9Mo(C5Me5)] in a single-crystal to single-crystal process by X-ray diffraction has been achieved for the first time. Density functional theory studies reveal that the hydrogen addition process is cooperatively assisted by the Y/Mo heteromultimetallic sites, thus offering unprecedented insight into the hydrogen addition and release process of a metal hydride cluster.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Adams, R. A. & Cotton, F. A. Catalysis by Di- and Polynuclear Metal Cluster Complexes (Wiley-VCH, 1998).
Doherty, S. Organometallic chemistry of bi- and poly-nuclear complexes. Annu. Rep. Prog. Chem. A 93, 395–432 (1996).
Suzuki, H. Activation of organic substrates on multi-metallic sites of transition metal polyhydride clusters having C5Me5 groups as auxiliary ligands. Eur. J. Inorg. Chem. 1009–1023 (2002).
Ohanessian, G. & Goddard, W. A. III. Valence-bond concepts in transition metals: metal hydride diatomic cations. Acc. Chem. Res. 23, 386–392 (1990).
Ephritikhine, M. Synthesis, structure, and reactions of hydride, borohydride, and aluminohydride compounds of the f-elements. Chem. Rev. 97, 2193–2242 (1997).
Hou, Z., Nishiura, M. & Shima, T. Synthesis and reactions of polynuclear polyhydrido rare earth metal complexes containing ‘(C5Me4SiMe3)LnH2’ units: a new frontier in rare earth metal hydride chemistry. Eur. J. Inorg. Chem. 2535–2545 (2007).
Kubas, G. J. Fundamentals of H2 binding and reactivity on transition metals underlying hydrogenase function and H2 production and storage. Chem. Rev. 107, 4152–4205 (2007).
Schlapbach, L. & Züttel, A. Hydrogen-storage materials for mobile applications. Nature 414, 353–358 (2001).
Takimoto, M. & Hou, Z. Hydrogen at the flick of a switch. Nature 443, 400–401 (2006).
Schüth, F., Bogdanović, B. & Felderhoff, M. Light metal hydrides and complex hydrides for hydrogen storage. Chem. Commun. 2249–2258 (2004).
Evans, W. J., Meadows, J. H. & Hanusa, T. P. Organolanthanide and organoyttrium hydride chemistry. 6. Direct synthesis and 1H NMR spectral analysis of the trimetallic yttrium and yttrium–zirconium tetrahydride complexes, {[(C5H5)2YH]3H}{Li(THF)4} and {[(CH3C5H4)2YH]2[(CH3C5H4)2ZrH]H}. J. Am. Chem. Soc. 106, 4454–4460 (1984).
Alvarez, D. Jr, Caulton, K. G., Evans, W. J. & Ziller, J. W. Synthesis, structure, and reactivity of heterometallic polyhydride complexes of rhenium with yttrium and lutetium. Inorg. Chem. 31, 5500–5508 (1992).
Alvarez, D., Caulton, K. G., Evans, W. J. & Ziller, J. W. Reversible opening and closing of hetero trimetallic units in (C5H5)2Y(THF)Re2H7(PMe2Ph)4 and (C5H5)2LuRe2H7(PMe2Ph)4 . J. Am. Chem. Soc. 112, 5674–5676 (1990).
Butovskii, M. V., Tok, O. L., Wagner, F. R. & Kempe, R. Bismetallocenes: lanthanoid–transition-metal bonds through alkane elimination. Angew. Chem. Int. Ed. 47, 6469–6472 (2008).
Shima, T. & Hou, Z. Rare earth/d-transition metal heteomultimetallic polyhydride complexes based on half-sandwich rare earth moieties. Organometallics 28, 2244–2252 (2009).
Shima, T. & Hou, Z. Activation and dehydrogenative silylation of the C–H bonds of phosphine-coordinated ruthenium in Lu/Ru heteromultimetallic hydride complexes. Chem. Lett. 37, 298–299 (2008).
Radu, N. S., Gantzel, P. K. & Tilley, T. D. Lanthanide–tungsten heterobimetallic complexes via σ-bond metathesis. J. Chem. Soc. Chem. Commun. 1175–1176 (1994).
Green, M. L. H., Hughes, A. K., Michaelidou, D. M. & Mountford, P. New lanthanide–hydrogen–transition metal compounds: [{(PMe3)3WH5}2Yb·L3] and [{(η-C5H5)2NbH2}2Yb·L3] where L3 = (MeOCH2CH2)2O. J. Chem. Soc. Chem. Commun. 591–593 (1993).
Cheng, J., Saliu, K., Ferguson, M. J., McDonald, R. & Takats, J. Variable nuclearity scorpionate-supported lanthanide polyhydrides: [(TpR,R')LnH2]n (n = 3, 4 and 6). J. Organometall. Chem. 695, 2696–2702 (2010).
Cheng, J., Ferguson, M. J. & Takats, J. Synthesis and reaction of [(TpiPr2)LnH2]3 (Ln = Y, Lu) with CO: trinuclear cluster-bound propenolate en route to selective formation of propene. J. Am. Chem. Soc. 132, 2–3 (2010).
Cheng, J. et al. Scorpionate-supported dialkyl and dihydride lanthanide complexes: ligand- and solvent-dependent cluster hydride formation. Angew. Chem. Int. Ed. 47, 4910–4913 (2008).
Ohashi, M. et al. Rare-earth metal alkyl and hydride complexes stabilized by a cyclen-derived [NNNN] macrocyclic ancillary ligand. J. Am. Chem. Soc. 130, 6920–6921 (2008).
Hultzsch, K. C., Voth, P., Spaniol, T. P. & Okuda, J. Synthesis and characterization of a tetranuclear hydride cluster of yttrium [{(η5-C5Me4SiMe3)Y}4(μ-H)4(μ3-H)4(THF)2]. Z. Anorg. Allg. Chem. 629, 1272–1276 (2003).
Lyubov, D. M., Döring, C., Ketkov, S. Y., Kempe, R. & Trifonov, A. A. Selective protonation of the Y–C bond in trinuclear yttrium alkyl–hydrido clusters and formation of the cationic polyhydrido core. Chem. Eur. J. 17, 3824–3826 (2011).
Lyubov, D. M. et al. Selective assembly of trinuclear rare-earth alkyl hydrido clusters supported by aminopyridinate ligands. Organometallics 27, 2905–2907 (2008).
Nishiura, M. & Hou, Z. Novel polymerization catalysts and hydride clusters from rare-earth metal dialkyls. Nature Chem. 2, 257–268 (2010).
Nishiura, M., Baldamus, J., Shima, T., Mori, K. & Hou, Z. Synthesis and structures of the C5Me4SiMe3-supported polyhydride complexes over the full size-range of the rare earth series. Chem. Eur. J. 17, 5033–5044 (2011).
Cheng, J., Shima, T. & Hou, Z. Rare-earth polyhydride complexes bearing bis(phosphinophenyl)amido pincer ligands. Angew. Chem. Int. Ed. 50, 1857–1860 (2011).
Shima, T. & Hou, Z. Selective ammonolysis of half-sandwich rare earth metal dialkyl and polyhydride complexes: synthesis of polyamido rare earth complexes having novel structures. Dalton Trans. 39, 6858–6863 (2010).
Takenaka, Y., Shima, T., Baldamus, J. & Hou, Z. Reduction of transition-metal-coordinated carbon monoxide by a rare-earth hydride cluster: isolation of well-defined heteromultimetallic oxycarbene, oxymethyl, carbene and methyl complexes. Angew. Chem. Int. Ed. 48, 7888–7891 (2009).
Yousufuddin, M. et al. Neutron diffraction studies on 4-coordinate hydrogen atom in an yttrium cluster. J. Am. Chem. Soc. 130, 3888–3891 (2008).
Li, X., Baldamus, J., Nishiura, M. & Hou, Z. Cationic rare-earth polyhydrido complexes: synthesis, structure, and catalytic activity for the cis-1,4-selective polymerization of 1,3-cyclohexadiene. Angew. Chem. Int. Ed. 45, 8184–8188 (2006).
Shima, T. & Hou, Z. Hydrogenation of carbon monoxide by tetranuclear rare earth metal polyhydrido complexes. Selective formation of ethylene and isolation of well-defined polyoxo rare earth metal clusters. J. Am. Chem. Soc. 128, 8124–8125 (2006).
Cui, D., Nishiura, M. & Hou, Z. Alternating copolymerization of cyclohexene oxide and carbon dioxide catalyzed by organo rare earth metal complexes. Macromolecules 38, 4089–4095 (2005).
Luo, Y., Baldamus, J., Tardif, O. & Hou, Z. DFT study of the tetranuclear lutetium and yttrium polyhydride cluster complexes [(C5Me4SiMe3)4Ln4H8] (Ln = Lu, Y) that contain a four-coordinate hydrogen atom. Organometallics 24, 4362–4366 (2005).
Cui, D., Nishiura, M. & Hou, Z. Lanthanide–imido complexes and their reactions with benzonitrile. Angew. Chem. Int. Ed. 44, 959–962 (2005).
Tardif, O., Hashizume, D. & Hou, Z. Hydrogenation of carbon dioxide and aryl isocyanates by a tetranuclear tetrahydrido yttrium complex. Isolation, structures, and CO2 insertion reactions of methylene diolate and μ3-oxo yttrium complexes. J. Am. Chem. Soc. 126, 8080–8081 (2004).
Cui, D., Tardif, O. & Hou, Z. Tetranuclear rare earth metal polyhydrido complexes composed of ‘(C5Me4SiMe3)LnH2’ units. Unique reactivities toward unsaturated C–C, C–N, and C–O bonds. J. Am. Chem. Soc. 126, 1312–1313 (2004).
Tardif, O., Nishiura, M. & Hou, Z. Isolation and structural characterization of a polyhydrido lanthanide cluster complex consisting of ‘(C5Me4SiMe3)LuH2’ units. Organometallics 22, 1171–1173 (2003).
Stewart, T. et al. The space between: neutron diffraction studies reveal multiple hydrogen atom coordination numbers in an anionic dysprosium hydride cluster. Inorg. Chim. Acta. 363, 562–566 (2010).
Shin, J. H. & Parkin, G. The syntheses, structures and reactivity of some mononuclear and dinuclear pentamethylcyclopentadienyl molybdenum complexes. Polyhedron 13, 1489–1493 (1994).
Bau, R. et al. Five-coordinated hydrogen: neutron diffraction analysis of the hydrido cluster complex [H2Rh13(CO)24]3–. Science 275, 1099–1102 (1997).
Collman, J. P., Hegedus, L. S., Norton, J. R. & Finke, R. G. Principles and Applications of Organotransition Metal Chemistry 279–353 (University Science Books, 1987).
Kawamichi, T., Haneda, T., Kawano, M. & Fujita, M. X-ray observation of a transient hemiaminal trapped in a porous network. Nature 461, 633–635 (2009).
Supriya, S. & Das, S. K. Reversible single crystal to single crystal transformation through Fe–O(H)Me/Fe–OH2 bond formation/bond breaking in a gas–solid reaction at an ambient condition. J. Am. Chem. Soc. 129, 3464–3465 (2007).
Kobatake, S., Takami, S., Muto, H., Ishikawa, T. & Irie, M. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 446, 778–781 (2007).
Zhang, J.-P., Lin, Y.-Y., Zhang, W.-X., & Chen, X.-M. Temperature- or guest-induced drastic single-crystal-to-single-crystal transformations of a nanoporous coordination polymer. J. Am. Chem. Soc. 127, 14162–14163 (2005).
Albrecht, M., Lutz, M., Spek, A. L. & van Koten, G. Organoplatinum crystals for gas-triggered switches. Nature 406, 970–974 (2000).
Tokitoh, N. et al. A unique crystalline-state reaction of an overcrowded distibene with molecular oxygen: the first example of a single crystal to a single crystal reaction with an external reagent. J. Am. Chem. Soc. 120, 433–434 (1998).
Ohashi, Y. & Sasada, Y. X-ray analysis of Co–C bond cleavage in the crystalline state. Nature 267, 142–144 (1977).
Huang, Z., White, P. S. & Brookhart, M. Ligand exchanges and selective catalytic hydrogenation in molecular single crystals. Nature 465, 598–601 (2010).
Ohno, K. & Maeda, S. A scaled hypersphere search method for the topography of reaction pathways on the potential energy surface. Chem. Phys. Lett. 384, 277–282 (2004).
Momma, K. & Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Cryst. 41, 653–658 (2008).
Kirtley, S. W., Olsen, J. P. & Bau, R. Location of the hydrogen atoms in H3Mn3(CO)12. A crystal structure determination. J. Am. Chem. Soc. 95, 4532–4536 (1973).
Acknowledgements
This work is dedicated to the memory of Professor Robert Bau (1944–2008). We are grateful to K. Aznavour for her assistance in initial neutron diffraction studies, to the Institut Laue-Langevin for the allocations of beam time on D19 and VIVALDI for the neutron studies quoted here of 2b and 3b , respectively, and to D. Hashizume for help with analysis of the neutron data of 3b . This work was supported by a Grant-in-Aid for Young Scientists (B) (no. 21750068), a Grant-in-Aid for Scientific Research (S) (no. 21225004) from JSPS, National Natural Science Foundation of China (no. 21028001), RICC (RIKEN Integrated Cluster of Clusters), and the Network and Information Centre of Dalian University of Technology for computational resources. Z.H. is grateful to the Chang Jiang Scholar Program for Visiting Professor.
Author information
Authors and Affiliations
Contributions
T. Shima carried out all syntheses, NMR and X-ray characterizations, and reactivity studies. Y.L. carried out DFT calculations. T. Stewart, R.B., G.J.M. and S.A.M. carried out neutron diffraction studies. Z.H. directed the project. T. Shima and Z.H. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5015 kb)
Supplementary information
Crystallographic data for compound 2a (CIF 37 kb)
Supplementary information
Crystallographic data data for compound 2b (CIF 39 kb)
Supplementary information
Neutron diffraction for compound 2b (CIF 65 kb)
Supplementary information
Crystallographic data for compound 3a (CIF 36 kb)
Supplementary information
Crystallographic data data for compound 3b (CIF 36 kb)
Supplementary information
Neutron diffraction for compound 3b (CIF 54 kb)
Supplementary information
Crystallographic data for compound 4 (CIF 35 kb)
Supplementary information
Crystallographic data for compound 5 (CIF 37 kb)
Supplementary information
Crystallographic data for compound 3a_Dy (CIF 37 kb)
Supplementary information
Crystallographic data for compound 5_Dy (CIF 37 kb)
Supplementary information
Crystallographic data for compound 3a_Ho (CIF 43 kb)
Supplementary information
Crystallographic data for compound 5_Ho (CIF 38 kb)
Rights and permissions
About this article
Cite this article
Shima, T., Luo, Y., Stewart, T. et al. Molecular heterometallic hydride clusters composed of rare-earth and d-transition metals. Nature Chem 3, 814–820 (2011). https://doi.org/10.1038/nchem.1147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1147
This article is cited by
-
Carbon–carbon bond cleavage and rearrangement of benzene by a trinuclear titanium hydride
Nature (2014)
-
An oxyhydride of BaTiO3 exhibiting hydride exchange and electronic conductivity
Nature Materials (2012)
-
Rare-earth dialkyl and dihydride complexes bearing monoanionic ancillary ligands
Science China Chemistry (2011)