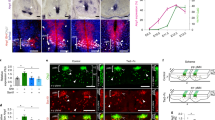
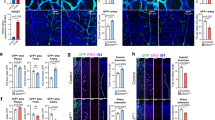
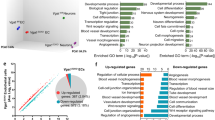
Abstract
The vasculature is a prominent component of the subventricular zone neural stem cell niche. Although quiescent neural stem cells physically contact blood vessels at specialized endfeet, the significance of this interaction is not understood. In contrast, it is well established that vasculature-secreted soluble factors promote lineage progression of committed progenitors. Here we specifically investigated the role of cell–cell contact-dependent signalling in the vascular niche. Unexpectedly, we find that direct cell–cell interactions with endothelial cells enforce quiescence and promote stem cell identity. Mechanistically, endothelial ephrinB2 and Jagged1 mediate these effects by suppressing cell-cycle entry downstream of mitogens and inducing stemness genes to jointly inhibit differentiation. In vivo, endothelial-specific ablation of either of the genes which encode these proteins, Efnb2 and Jag1 respectively, aberrantly activates quiescent stem cells, resulting in depletion. Thus, we identify the vasculature as a critical niche compartment for stem cell maintenance, furthering our understanding of how anchorage to the niche maintains stem cells within a pro-differentiative microenvironment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Fuchs, E., Tumbar, T. & Guasch, G. Socializing with the neighbors: stem cells and their niche. Cell 116, 769–778 (2004).
Chen, S., Wang, S. & Xie, T. Restricting self-renewal signals within the stem cell niche: multiple levels of control. Curr. Opin. Genet. Dev. 21, 684–689 (2011).
Wagers, A. J. The stem cell niche in regenerative medicine. Cell Stem Cell 10, 362–369 (2012).
Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Ihrie, R. A. & Alvarez-Buylla, A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 70, 674–686 (2011).
Garcia-Verdugo, J. M., Doetsch, F., Wichterle, H., Lim, D. A. & Alvarez-Buylla, A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J. Neurobiol. 36, 234–248 (1998).
Mirzadeh, Z., Merkle, F. T., Soriano-Navarro, M., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3, 265–278 (2008).
Tavazoie, M. et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3, 279–288 (2008).
Shen, Q. et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell–cell interactions. Cell Stem Cell 3, 289–300 (2008).
Shen, Q. et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 (2004).
Kokovay, E. et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signalling. Cell Stem Cell 7, 163–173 (2010).
Calvo, C. F. et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 25, 831–844 (2011).
Quaegebeur, A., Lange, C. & Carmeliet, P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron 71, 406–424 (2011).
Goldberg, J. S. & Hirschi, K. K. Diverse roles of the vasculature within the neural stem cell niche. Regen. Med. 4, 879–897 (2009).
Chen, S., Lewallen, M. & Xie, T. Adhesion in the stem cell niche: biological roles and regulation. Development 140, 255–265 (2013).
Pasquale, E. B. Eph-ephrin bidirectional signalling in physiology and disease. Cell 133, 38–52 (2008).
Pierfelice, T., Alberi, L. & Gaiano, N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69, 840–855 (2011).
Imayoshi, I., Sakamoto, M., Yamaguchi, M., Mori, K. & Kageyama, R. Essential roles of Notch signalling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 30, 3489–3498 (2010).
Ehm, O. et al. RBPJkappa-dependent signalling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J. Neurosci. 30, 13794–13807 (2010).
Nomura, T., Goritz, C., Catchpole, T., Henkemeyer, M. & Frisen, J. EphB signalling controls lineage plasticity of adult neural stem cell niche cells. Cell. Stem Cell 7, 730–743 (2010).
Theveneau, E. & Mayor, R. Collective cell migration of epithelial and mesenchymal cells. Cell Mol. Life Sci. 70, 3481–3492 (2013).
Pastrana, E., Cheng, L. C. & Doetsch, F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc. Natl Acad. Sci. USA 106, 6387–6392 (2009).
Wurmser, A. E. et al. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 430, 350–356 (2004).
Hack, M. A. et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat. Neurosci. 8, 865–872 (2005).
Menn, B. et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 26, 7907–7918 (2006).
Yoshioka, N., Asou, H., Hisanaga, S. & Kawano, H. The astrocytic lineage marker calmodulin-regulated spectrin-associated protein 1 (Camsap1): phenotypic heterogeneity of newly born Camsap1-expressing cells in injured mouse brain. J. Comp. Neurol. 520, 1301–1317 (2012).
Sherr, C. J. D-type cyclins. Trends Biochem. Sci. 20, 187–190 (1995).
Miao, H. et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat. Cell Biol. 3, 527–530 (2001).
Parrinello, S. et al. EphB signalling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 143, 145–155 (2010).
Gale, N. W. et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev. Biol. 230, 151–160 (2001).
Koch, U., Lehal, R. & Radtke, F. Stem cells living with a Notch. Development 140, 689–704 (2013).
Zhu, T. S. et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 71, 6061–6072 (2011).
Andreu-Agullo, C., Morante-Redolat, J. M., Delgado, A. C. & Farinas, I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat. Neurosci. 12, 1514–1523 (2009).
Mizutani, K., Yoon, K., Dang, L., Tokunaga, A. & Gaiano, N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 (2007).
Kawaguchi, D., Furutachi, S., Kawai, H., Hozumi, K. & Gotoh, Y. Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nat. Commun. 4, 1880 (2013).
Kokovay, E. et al. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell 11, 220–230 (2012).
Doetsch, F., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046–5061 (1997).
Miller, F. D. & Gauthier-Fisher, A. Home at last: neural stem cell niches defined. Cell Stem Cell 4, 507–510 (2009).
Conover, J. C. et al. Disruption of Eph/ephrin signalling affects migration and proliferation in the adult subventricular zone. Nat. Neurosci. 3, 1091–1097 (2000).
Mathieu, C. et al. Endothelial cell-derived bone morphogenetic proteins control proliferation of neural stem/progenitor cells. Mol. Cell. Neurosci. 38, 569–577 (2008).
Sun, Y., Hu, J., Zhou, L., Pollard, S. M. & Smith, A. Interplay between FGF2 and BMP controls the self-renewal, dormancy and differentiation of rat neural stem cells. J. Cell Sci. 124, 1867–1877 (2011).
Codega, P. et al. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 82, 545–559 (2014).
Crosnier, C., Stamataki, D. & Lewis, J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet. 7, 349–359 (2006).
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010).
Brooker, R., Hozumi, K. & Lewis, J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 133, 1277–1286 (2006).
Grunwald, I. C. et al. Hippocampal plasticity requires postsynaptic ephrinBs. Nat. Neurosci. 7, 33–40 (2004).
Lim, D. A. & Alvarez-Buylla, A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl Acad. Sci. USA 96, 7526–7531 (1999).
Scheffler, B. et al. Phenotypic and functional characterization of adult brain neuropoiesis. Proc. Natl Acad. Sci. USA 102, 9353–9358 (2005).
Pollard, S. et al. Adherent Neural Stem (NS) cells from fetal and adult forebrain. Cereb. Cortex 16, i112–i120 (2006).
Ferron, S. R. et al. A combined ex/in vivo assay to detect effects of exogenously added factors in neural stem cells. Nat. Protoc. 2, 849–859 (2007).
Kintner, C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell 69, 225–236 (1992).
Davy, A. & Soriano, P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev. Biol. 304, 182–193 (2007).
Mirzadeh, Z., Doetsch, F., Sawamoto, K., Wichterle, H. & Alvarez-Buylla, A. The subventricular zone en-face: wholemount staining and ependymal flow. J. Vis. Exp. 39, 1938 (2010).
Acknowledgements
This work was funded by the Medical Research Council, UK (C.O., B.K., M.C. and S.P.) and the Royal Society (S.P.). We thank A. Fisher, M. Raff, L. Aragon and M. Ungless for critical reading of the manuscript, S. Nicolis (Univerity of Milano-Bicocca, Italy), M. Herlyn (The Wistar Institute, USA) and J. Gil (MRC Clinical Sciences Centre, UK) for constructs, S. Di Giovanni (Imperial College London, UK) and V. Taylor (University of Basel, Switzerland) for Hes5–GFP neural stem cells, S. Pollard (MRC Centre for Regenerative Medicine, UK) for cells, T. Makinen (University of Uppsala, Sweden) for ephrinB2 antibody and J. L. Tremoleda for technical advice.
Author information
Authors and Affiliations
Contributions
C.O. helped design the project, performed most of the experiments, analysed the data and helped prepare the manuscript. B.K. performed animal injections and contributed to experiments throughout the study. A.W. performed quantifications and helped with experiments. M.C. performed animal injections. M.E.P. and R.H.A. provided Rbpjflox/flox tissue and characterized Efnb2–GFP reporter mice. G.Q. generated aNSC neurosphere cultures. S.P. designed and supervised the project, analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Endothelial cells promote a type-B-like phenotype in adult neural stem cells.
(a) Representative immunofluorescence images and quantification of active caspase 3 staining of untreated NPC cultured alone or with endothelial cells for 24 h or bleomycin-treated NPC monocultures as positive control. n = 2 independent experiments each pooled from duplicate dishes. A minimum of 200 cells per condition per experiment was counted. (b) Representative immunofluorescence images and quantifications of control NPCs cultured in the absence or presence of endothelial cells and DN-N-cadherin overexpressing neural precursor cells cultured with endothelial cells, as stated. Cultures were stained with N-cadherin (green) and nuclei counterstained with DAPI (Blue). Quantifications were performed as in a (n = 2 dishes from independent experiments). (c) Quantification of cell cycle profiles measured by FACS of NPCs cultured alone (−), in direct (pEC) or transwell (+Sol) coculture with pulmonary endothelial cells (pEC, n = 3 independent experiments performed on single dishes). Two-tailed paired Student’s t-test. (d) Quantification of cell cycle profiles of aNSC measured by FACS cultured alone (−), in transwell (+Sol) or direct coculture with immortalized (bEND) or primary (bmvECs) brain microvascular endothelial cells (n = 3 independent experiments performed on single dishes). Two-tailed paired Student’s t-test. (e) Quantification of cell cycle profiles measured by FACS. NPCs were cultured alone (−), at high cell density (NPC high den), with endothelial cells at low density (+bEND low den), or with endothelial cells at high density after overexpression of DN-N-cadherin constructs and pulsed with BrdU for 1 h prior to analysis (n = 3 independent experiments performed on single dishes). One-way ANOVA with Bonferroni correction. (f) Quantitative RT-PCR analysis of mRNA levels of type-B and type-C marker genes in NPC cultured alone or with pEC. Bar graphs represent fold change relative to control (n = 3 independent experiments performed on single dishes). Two-tailed paired Student’s t-test. (g) Quantitative RT-PCR analysis of mRNA levels of type-B and type-C marker genes in aNSC cultured alone or with bEND or bmvECs (n = 3). Two-tailed paired Student’s t-test. (h) Representative immunofluorescence images of Td-Tomato labelled-NPC seeded alone (−) or in coculture with pEC for 24h and either left unstained to assess NPC morphology, or stained for the indicated type-B and type-C markers. (i) Representative FACS profiles and quantification of EGFR+ wildtype NPC and GFP+ Hes5-GFP reporter NPCs in the same culture conditions as in (h). (j) Representative immunofluorescence images of aNSC seeded alone (aNSC) or in coculture with bEND3 for 24h and stained for the indicated markers (k) Representative FACS profiles and quantification of EGFR+ wildtype aNSC and GFP+ Hes5-GFP reporter aNSC in the same culture conditions as in (j). (l) Quantification of cell cycle profiles measured by FACS of aNSC treated with control proteins (Fc) or recombinant ephrinB2 and Jagged1 ligands (n = 3 independent experiments performed on single dishes). Two-tailed paired Student’s t-test. (m) Quantitative RT-PCR analysis of mRNA levels of type-B and type-C marker genes in aNSC treated with indicated recombinant proteins (n = 3 RNA extracts from independent experiments). Two-tailed paired Student’s t-test. Numbers within all pictures indicate the percentage of marker positive cells in each condition. For this and all later Supplementary figures ∗∗∗ = p < 0.001, ∗∗ = p < 0.01, ∗ = p < 0.05. All bars represent mean ± s.e.m. Each dot represents an independent experiment. Source data in Supplementary Table 1. Scale bars: (a, b) = 20 μm; (h, j) = 25 μm.
Supplementary Figure 2 Co-culture with endothelial cells inhibits NPC differentiation and maintains stemness.
(a) FACS analysis for the endothelial markers CD31 and VE-Cadherin of cell-tracker-labelled NPCs cultured alone, unlabelled bEND cultured alone or cocultured NPC and bEND for 4d in differentiating conditions. Note the absence of CD31+ or VE-Cadherin+ NPCs in the cocultures and retention of endothelial marker expression in bEND. (b) Following 4d coculture with bEND in differentiation conditions, NPCs were separated from the endothelial monolayer and differentiated. Shown is a representative fluorescent image of differentiated cultures stained for Tuj1, GFAP (green), O4 (red) and DAPI (blue). (c) Representative immunofluorescence images and quantification of aNSC differentiated for 4d on their own or in co-culture with bEND and stained for the indicated differentiation markers (n = 3 independent experiments pooled from duplicate dishes). A minimum of 200 cells counted across randomly selected fields of view were counted per condition per experiment. Error bars denote s.e.m. Two-tailed paired Student’s t-test. (d) Quantitative RT-PCR of NPC cultured alone (−), in transwell (+Sol) or direct co-culture with endothelial cells (+bEND) and induced to differentiate for 4 days. Average expression levels of representative type-B marker genes and genes associated with differentiation along the oligondendrocytic (Olig2) and astrocytic (CAMSAP1, AQP4) lineages are shown (n = 3 independent experiments performed on single dishes). Error bars denote s.e.m. One-way ANOVA with Bonferroni correction. (e) western analysis for GFAP and Tuj1 of the same cultures as in (d). Each dot represents an independent experiment. Source data in Supplementary Table 1. Scale bars: (b) = 15 μm; (c) = 25 μm.
Supplementary Figure 3 ephrinB2 inhibits proliferation through ERK/CyclinD1 but regulates type-B identity independently of ERK.
(a) Quantification of cell cycle profiles measured by FACS of NPCs left untreated or treated with U0126 for 24h (n = 3 independent experiments performed on single dishes). Two-tailed paired Student’s t-test. (b) Quantification of cell cycle profiles measured by FACS. NPC were infected with CyclinD1-encoding retroviral vectors (pBabe-CyclinD1) or empty control vectors (pBabe) and cocultured with bEND or treated with ephrinB2-Fc ligands for 24h (n = 2 independent experiments performed on single dishes). (c) Quantitative RT-PCR analysis of neural precursor cells seeded on control Fc or EphrinB2-Fc for 24h for the indicated type-B marker genes. Note that ephrinB2 only significantly upregulates GFAP. (n = 3 independent experiments performed on single dishes). Error bars denote s.d. Two-tailed paired Student’s t-test. (d) Representative immunofluorescence image of Fc or ephrinB2-Fc treated NPC stained for GFAP (red) and Sox2 (green). Scale bar = 25μm. (e) Quantitative RT-PCR analysis for the indicated type-B and type-C marker genes and CyclinD1 of NPCs left untreated (Ctl) or treated with U0126 for 24h (n = 6 independent experiments performed on single dishes). Two-tailed paired Student’s t-test. (f) Western analysis of ephrinB2 levels in wild type and efnb2−/− pEC to confirm efficiency of efnb2 deletion. β-tubulin served as loading control. Each dot represents an independent experiment. All bars represent mean ± s.e.m. unless otherwise specified. Source data in Supplementary Table 1.
Supplementary Figure 4 Jagged-1 promotes type-B fate and has a minor effect on NPC proliferation.
(a) Quantitative RT-PCR analysis of mRNA levels of stated Notch target genes and CyclinD1 in NPC cultured alone (−) or with endothelial cells (+bEND) in the absence (DMSO) or presence of the Notch inhibitor DAPT (n = 3 independent experiments performed on single dishes). One-way ANOVA with Bonferroni correction. (b) Quantitative RT-PCR analysis for the Notch target genes Hes5 and Hey2 in NPC infected with control or DN-N-cadherin vectors and cultured on their own or with endothelial cells, as indicated. (n = 2 independent experiments performed on single dishes). (c) Western blot showing efficiency of Jagged1 knock-down in endothelial cells and loading control (□□□□□). (d) Quantitative RT-PCR analysis of the indicated Notch target genes in NPC cultured in the absence (−) or presence of Scr siRNA-treated endothelial cells and in the presence of endothelial cells knocked down for Jagged1 (Jag1) using a second independent oligo to the one shown in Fig. 4d. (n = 2 independent experiments performed on single dishes). (e) Western analysis of NICD in Fc and Jagged1-Fc treated NPCs, β-tubulin served as loading control. (f) Quantification of cell cycle profiles measured by FACS of NPCs cocultured with scramble siRNA-transfected (scr) bEND or bEND knock-down for Jagged1 (Jag1) with two independent oligos (n = 2 independent experiments per oligo performed on single dishes). Two-tailed paired Student’s t-test. Each dot represents an independent experiment. All bars represent mean ± s.e.m. Source data in Supplementary Table 1.
Supplementary Figure 5 Eph and Notch signalling do not crosstalk.
(a) Quantification of cell cycle profiles measured by FACS of NPCs treated with Fc, ephrinB2-Fc, Jagged1-Fc or both for 24h (n = 3 independent experiments performed on single dishes). One-way ANOVA with Bonferroni correction. (b) Quantification of cell cycle profiles measured by FACS of NPC cocultured with efnb2−/− pEC in the absence or presence of DAPT (n = 3 independent experiments performed on single dishes). Two-tailed paired Student’s t-test. (c) Quantitative RT-PCR analysis of mRNA levels of the indicated ephrinB2- (green) and jagged1-regulated (blue) type-B and type-C marker genes in NPC treated with both ligands (orange) relative to treatment with the respective single ligand (n = 3 independent experiments performed on single dishes). Two-tailed paired Student’s t-test. (d) Western blot analysis of p-ERK and total ERK levels in NPC treated with Fc or ephrinB2-Fc ligands in differentiation conditions for 4d. (e) Quantification of neurons (Tuj1+), oligodendrocytes (O4+), astrocytes (GFAP+/Sox2) and type-B-like stem cells (GFAP+/Sox2+) in NPCs cultured in differentiation conditions and treated with U0126 at d3 and 4 in the absence or presence of Jagged1 ligands as indicated. Shown is one representative experiment performed on duplicate dishes of three experiments that gave similar results. A minimum of 200 cells counted across randomly selected fields of view were counted per condition per experiment. One-way ANOVA with Bonferroni correction. All bars represent mean ± s.e.m. Source data in Supplementary Table 1.
Supplementary Figure 6 ephrin-B2 and Jagged-1 ligands are expressed in adult SVZ vessels.
(a) Vascular specificity of Cdh5CreER-induced recombination. ROSA26-YFP reporter expression and CD31 staining in the SVZ of ROSA26-YFP;Cdh5CreER mice injected with tamoxifen postnatally and imaged 2 weeks later. (b) Immunofluorescence staining for ephrinB2 (red) and the endothelial marker CD31 (green) of coronal sections of the SVZ of Efnb2iΔEC and tamoxifen injected Efnb2flox control littermates (Ctl). (c) Immunofluorescence staining for Jagged-1 (red) and the endothelial marker CD31 (green) of coronal sections of the SVZ of Jag1iΔEC and tamoxifen injected Jag1flox control littermate. No primary control is shown in the right. Nuclei were counterstained with DAPI. (d) Immunofluorescence analysis for NICD (green) in the SVZ of Ctl and Jag1iΔEC mice 4 weeks after tamoxifen injection, (e) Immunofluorescence analysis for GFAP and S100β of coronal sections of the SVZ of Efnb2iΔEC and Jag1iΔEC mice and respective controls at 4 weeks post-recombination. The absence of GFAP+/S100β+ cells in the mutant brains confirms absence of abnormal astrogliosis. (f) Quantification of activated Caspase3+ cells in the olphactory bulbs of Ctl, Efnb2iΔEC and Jag1iΔEC mice (n = 3 for all genotypes). Error bars denote s.e.m. Each dot represents a mouse. Two-tailed paired Student’s t-test. (g, h) Representative images and quantification of the percentages of neuronal marker NeuN+ EdU label retaining cells in the olphactory bulb (all Efnb2iΔEC n = 3, all Jag1iΔEC n = 2). Note that the vast majority of EdU label retaining cells in the olfactory bulb are newborn neurons in all genotypes. Mean ± s.e.m. Each dot represents a mouse. Two-tailed paired Student’s t-test. Source data in Supplementary Table 1. Scale bars: (a) = 40μm, (b) = 15μm, (c) = 25μm, (d) = 20μm, (e) = 25μm, (g) = 50μm.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2402 kb)
Supplementary Table 1
Supplementary Information (XLSX 88 kb)
Supplementary Table 2
Supplementary Information (XLSX 37 kb)
GFP-labelled NPC cultured alone.
Time-lapse microscopy of GFP-overexpressing neural precursor cells cultured in growth media. Frames were taken every 10 min for 48h. Only GFP fluorescence is shown. (MOV 3411 kb)
GFP-labelled NPC cocultured with unlabelled endothelial cells.
Time-lapse microscopy of GFP-overexpressing neural precursor cells cocultured with endothelial cells in growth media. Frames were taken every 10 min for 48h. Only GFP fluorescence is shown. (MOV 3340 kb)
Rights and permissions
About this article
Cite this article
Ottone, C., Krusche, B., Whitby, A. et al. Direct cell–cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol 16, 1045–1056 (2014). https://doi.org/10.1038/ncb3045
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3045
This article is cited by
-
Diverse WGBS profiles of longissimus dorsi muscle in Hainan black goats and hybrid goats
BMC Genomic Data (2023)
-
Seasonal remodeling of the progenitor pool and its distribution in the ewe mediobasal hypothalamus
Cell and Tissue Research (2023)
-
Endothelial Rbpj Is Required for Cerebellar Morphogenesis and Motor Control in the Early Postnatal Mouse Brain
The Cerebellum (2022)
-
Angiocrine polyamine production regulates adiposity
Nature Metabolism (2022)
-
Angiocrine endothelium: from physiology to cancer
Journal of Translational Medicine (2020)