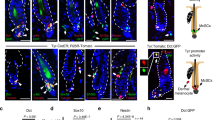
Abstract
Giant congenital naevi are pigmented childhood lesions that frequently lead to melanoma, the most aggressive skin cancer. The mechanisms underlying this malignancy are largely unknown, and there are no effective therapies. Here we describe a mouse model for giant congenital naevi and show that naevi and melanoma prominently express Sox10, a transcription factor crucial for the formation of melanocytes from the neural crest. Strikingly, Sox10 haploinsufficiency counteracts NrasQ61K-driven congenital naevus and melanoma formation without affecting the physiological functions of neural crest derivatives in the skin. Moreover, Sox10 is also crucial for the maintenance of neoplastic cells in vivo. In human patients, virtually all congenital naevi and melanomas are SOX10 positive. Furthermore, SOX10 silencing in human melanoma cells suppresses neural crest stem cell properties, counteracts proliferation and cell survival, and completely abolishes in vivo tumour formation. Thus, SOX10 represents a promising target for the treatment of congenital naevi and melanoma in human patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Bauer, J., Curtin, J. A., Pinkel, D. & Bastian, B. C. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J. Invest. Dermatol. 127, 179–182 (2007).
Ghosh, P. & Chin, L. Genetics and genomics of melanoma. Expert. Rev. Dermatol. 4, 131–143 (2009).
Ichii-Nakato, N. et al. High frequency of BRAFV600E mutation in acquired nevi and small congenital nevi, but low frequency of mutation in medium-sized congenital nevi. J. Invest. Dermatol. 126, 2111–2118 (2006).
Ackermann, J. et al. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 65, 4005–4011 (2005).
Serrano, M. et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85, 27–37 (1996).
Britsch, S. et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66–78 (2001).
Sommer, L. Generation of melanocytes from neural crest cells. Pigment Cell Melanoma Res. 24, 411–421 (2011).
Wong, C. E. et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J. Cell Biol. 175, 1005–1015 (2006).
Kuhlbrodt, K. et al. Functional analysis of Sox10 mutations found in human Waardenburg-Hirschsprung patients. J. Biol. Chem. 273, 23033–23038 (1998).
Civenni, G. et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 71, 3098–3109 (2011).
Nonaka, D., Chiriboga, L. & Rubin, B. P. Sox10: a pan-schwannian and melanocytic marker. Am. J. Surg. Pathol. 32, 1291–1298 (2008).
Bakos, R. M. et al. Nestin and SOX9 and SOX10 transcription factors are coexpressed in melanoma. Exp. Dermatol. 19, e89–e94 (2010).
Agnarsdottir, M. et al. SOX10 expression in superficial spreading and nodular malignant melanomas. Melanoma Res. 20, 468–478 (2010).
Flammiger, A. et al. SOX9 and SOX10 but not BRN2 are required fornestin expression in human melanoma cells. J. Invest. Dermatol. 129, 945–953 (2009).
Mihic-Probst, D. et al. Consistent expression of the stem cell renewal factor BMI-1 in primary and metastatic melanoma. Int. J. Cancer 121, 1764–1770 (2007).
Arnheiter, H. The discovery of the microphthalmia locus and its gene. Mitf. Pigment Cell Melanoma Res. 23, 729–735 (2010).
Paratore, C., Goerich, D. E., Suter, M. & Sommer, L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development 128, 3949–3961 (2001).
Herbarth, B. et al. Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc. Natl Acad. Sci. USA 95, 5161–5165 (1998).
Paratore, C., Eichenberger, C., Suter, U. & Sommer, L. Sox10 haploinsufficiency affects maintenance of progenitor cells in a mouse model of Hirschsprung disease. Hum. Mol. Genet. 11, 3075–3085 (2002).
Bondurand, N. et al. A molecular analysis of the yemenite deaf-blind hypopigmentation syndrome: SOX10 dysfunction causes different neurocristopathies. Hum. Mol. Genet. 8, 1785–1789 (1999).
Pingault, V. et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet. 18, 171–173 (1998).
Southard-Smith, E. M. et al. The Sox10(Dom) mouse: Modeling the genetic variation of Waardenburg- Shah (WS4) syndrome. Genome Res. 9, 215–225 (1999).
Lang, D. et al. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature 433, 884–887 (2005).
Nishimura, E. K. et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854–860 (2002).
Rabbani, P. et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 145, 941–955 (2011).
Kim, J., Lo, L., Dormand, E. & Anderson, D. J. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38, 17–31 (2003).
Bondurand, N., Natarajan, D., Barlow, A., Thapar, N. & Pachnis, V. Maintenance of mammalian enteric nervous system progenitors by SOX10 and endothelin 3 signalling. Development 133, 2075–2086 (2006).
John, N., Cinelli, P., Wegner, M. & Sommer, L. Transforming growth factor beta-mediated Sox10 suppression controls mesenchymal progenitor generation in neural crest stem cells. Stem Cells 29, 689–699 (2011).
Finzsch, M. et al. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J. Cell Biol. 189, 701–712 (2010).
Stolt, C. C., Lommes, P., Hillgartner, S. & Wegner, M. The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Res. 36, 5427–5440 (2008).
Boiko, A. D. et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 466, 133–137 (2010).
Bosenberg, M. et al. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis 44, 262–267 (2006).
Hoek, K. S. et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 19, 290–302 (2006).
Acknowledgements
We thank the FACS facility and the Functional Genomic Center Zurich (FGCZ) of the University of Zurich for technical assistance, S. Behnke and C. Burger for assistance in histology, and M. Serrano and L. Chin for providing mouse lines. This work was supported by the Swiss Cancer League (including a grant supporting a Collaborative Cancer Research Project (CCRP) by D.M.P., R.D. and L.S.), the Swiss National Science Foundation, the National Research Program (NRP63) ‘Stem Cells and Regenerative Medicine’ and a stipend from the UBS Wealth Management.
Author information
Authors and Affiliations
Contributions
O.S., P.C. and L.S. designed the experiments, O.S., P.C., D.Z., S.M.S., L.H., G.C., S.C. and J.B. performed the experiments, and O.S., P.C., M.O., D.M., R.D., Y.B., P.C. and L.S. analysed the data. R.D. provided human congenital naevus samples. D.M. and H.M. provided tissue microarrays of primary and metastatic melanoma samples. M.W. provided Sox10LacZ/+ and Sox10fl/+ mice. F.B. provided Tyr::NrasQ61K mice. O.S. and L.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1486 kb)
Supplementary Table 1
Supplementary Information (XLSX 46 kb)
Rights and permissions
About this article
Cite this article
Shakhova, O., Zingg, D., Schaefer, S. et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cell Biol 14, 882–890 (2012). https://doi.org/10.1038/ncb2535
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2535
This article is cited by
-
SOX10 deficiency-mediated LAMB3 upregulation determines the invasiveness of MAPKi-resistant melanoma
Oncogene (2024)
-
Functional analysis of recurrent CDC20 promoter variants in human melanoma
Communications Biology (2023)
-
Reactivation of embryonic genetic programs in tissue regeneration and disease
Nature Genetics (2023)
-
Treatment for giant congenital nevi moves a step closer
Cell Research (2022)
-
Targeting SOX10-deficient cells to reduce the dormant-invasive phenotype state in melanoma
Nature Communications (2022)