Abstract
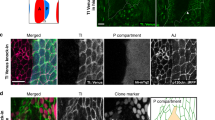
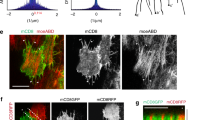
Partitioning tissues into compartments that do not intermix is essential for the correct morphogenesis of animal embryos and organs1,2,3. Several hypotheses have been proposed to explain compartmental cell sorting, mainly differential adhesion1,2,3,4,5,6,7,8,9, but also regulation of the cytoskeleton10,11 or of cell proliferation10,12. Nevertheless, the molecular and cellular mechanisms that keep cells apart at boundaries remain unclear. Here we demonstrate, in early Drosophila melanogaster embryos, that actomyosin-based barriers stop cells from invading neighbouring compartments. Our analysis shows that cells can transiently invade neighbouring compartments, especially when they divide, but are then pushed back into their compartment of origin. Actomyosin cytoskeletal components are enriched at compartmental boundaries, forming cable-like structures when the epidermis is mitotically active. When MyoII (non-muscle myosin II) function is inhibited, including locally at the cable by chromophore-assisted laser inactivation (CALI)13,14, in live embryos, dividing cells are no longer pushed back, leading to compartmental cell mixing. We propose that local regulation of actomyosin contractibility, rather than differential adhesion, is the primary mechanism sorting cells at compartmental boundaries.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dahmann, C. & Basler, K. Compartment boundaries: at the edge of development. Trends Genet. 15, 320–326 (1999).
Irvine, K. D. & Rauskolb, C. Boundaries in development: formation and function. Annu. Rev. Cell Dev. Biol. 17, 189–214 (2001).
Tepass, U., Godt, D. & Winklbauer, R. Cell sorting in animal development: signalling and adhesive mechanisms in the formation of tissue boundaries. Curr. Opin. Genet. Dev. 12, 572–582 (2002).
Dahmann, C. & Basler, K. Opposing transcriptional outputs of Hedgehog signaling and Engrailed control compartmental cell sorting at the Drosophila A/P boundary. Cell 100, 411–422 (2000).
Garcia-Bellido, A., Ripoll, P. & Morata, G. Developmental compartmentalisation of the wing disk of Drosophila. Nature New Biol. 245, 251–253 (1973).
Inoue, T. et al. Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development 128, 561–569 (2001).
Milan, M., Weihe, U., Perez, L. & Cohen, S. M. The LRR proteins Capricious and Tartan mediate cell interactions during DV boundary formation in the Drosophila wing. Cell 106, 785–794 (2001).
Schlichting, K., Demontis, F. & Dahmann, C. Cadherin Cad99C is regulated by Hedgehog signaling in Drosophila. Dev. Biol. 279, 142–154 (2005).
Vegh, M. & Basler, K. A genetic screen for hedgehog targets involved in the maintenance of the Drosophila anteroposterior compartment boundary. Genetics 163, 1427–1438 (2003).
Major, R. J. & Irvine, K. D. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development 132, 3823–3833 (2005).
Major, R. J. & Irvine, K. D. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev. Dyn. 235, 3051–3058 (2006).
O'Brochta, D. A. & Bryant, P. J. A zone of non-proliferating cells at a lineage restriction boundary in Drosophila. Nature 313, 138–141 (1985).
Jacobson, K., Rajfur, Z., Vitriol, E. & Hahn, K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 18, 443–450 (2008).
Jay, D. G. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc. Natl Acad. Sci. USA 85, 5454–5458 (1988).
Davy, A., Bush, J. O. & Soriano, P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 4, 1763–1776 (2006).
Perez-Pomares, J. M. & Foty, R. A. Tissue fusion and cell sorting in embryonic development and disease: biomedical implications. Bioessays 28, 809–821 (2006).
Twigg, S. R. et al. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc. Natl Acad. Sci. USA 101, 8652–8657 (2004).
Blair, S. S. & Ralston, A. Smoothened-mediated Hedgehog signalling is required for the maintenance of the anterior-posterior lineage restriction in the developing wing of Drosophila. Development 124, 4053–4063 (1997).
Cheng, Y. C. et al. Notch activation regulates the segregation and differentiation of rhombomere boundary cells in the zebrafish hindbrain. Dev. Cell 6, 539–550 (2004).
Cooke, J. E., Kemp, H. A. & Moens, C. B. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr. Biol. 15, 536–542 (2005).
Mellitzer, G., Xu, Q. & Wilkinson, D. G. Eph receptors and ephrins restrict cell intermingling and communication. Nature 400, 77–81 (1999).
Milan, M. & Cohen, S. M. A re-evaluation of the contributions of Apterous and Notch to the dorsoventral lineage restriction boundary in the Drosophila wing. Development 130, 553–562 (2003).
Morata, G. & Lawrence, P. A. Control of compartment development by the engrailed gene in Drosophila. Nature 255, 614–617 (1975).
Rodriguez, I. & Basler, K. Control of compartmental affinity boundaries by hedgehog. Nature 389, 614–618 (1997).
Shen, J. & Dahmann, C. The role of Dpp signaling in maintaining the Drosophila anteroposterior compartment boundary. Dev. Biol. 279, 31–43 (2005).
Xu, Q., Mellitzer, G., Robinson, V. & Wilkinson, D. G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature 399, 267–271 (1999).
Sanson, B. Generating patterns from fields of cells: examples from Drosophila segmentation. EMBO Rpts 2, 1083–1088 (2001).
Vincent, J. P. & O'Farrell, P. H. The state of engrailed expression is not clonally transmitted during early Drosophila development. Cell 68, 923–931 (1992).
Zallen, J. A. & Wieschaus, E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6, 343–355 (2004).
Rajfur, Z., Roy., P., Otey, C., Romer, L. & Jacobson, K. Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nature Cell Biol. 4, 286–293 (2002).
Diefenbach, T. J. et al. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J. Cell Biol. 158, 1207–1217 (2002).
Wang, F. S., Wolenski, J. S., Cheney, R. E., Mooseker, M. S. & Jay, D. G. Function of myosin-V in filopodial extension of neuronal growth cones. Science 273, 660–663 (1996).
Horstkotte, E., Schroder, T., Niewohner, J., Jay, D. G. & Henning, S. W. Toward understanding the mechanism of chromophore-assisted laser inactivation - evidence for the primary photochemical steps. Photochem. Photobiol. 81, 358–366 (2005).
Liao, J. C., Roider, J. & Jay, D. G. Chromophore-assisted laser inactivation of proteins is mediated by the photogeneration of free-radicals. Proc. Natl Acad. Sci. USA 91, 2659–2663 (1994).
Yan, P. et al. Fluorophore-assisted light inactivation of Calmodulin involves singlet-oxygen mediated cross-linking and methionine oxidation. Biochemistry 45, 4736–4748 (2006).
Laplante, C. & Nilson, L. A. Differential expression of the adhesion molecule Echinoid drives epithelial morphogenesis in Drosophila. Development 133, 3255–3264 (2006).
Wei, S. Y. et al. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev. Cell 8, 493–504 (2005).
Huber, A. B., Kolodkin, A. L., Ginty, D. D. & Cloutier, J.-F.o. Signaling at the growth cone: ligand–receptor complexes and the control of axon growth and guidance. Ann. Rev. Neurosci. 26, 509–563 (2003).
Townes, P. L. & Holtfreter, J. Directed movements and selective adhesion of embryonic amphibian cells. J. Exp. Zool. 128, 53–120 (1955).
Krieg, M. et al. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biol. 10, 429–436 (2008).
Royou, A., Sullivan, W. & Karess, R. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J. Cell Biol. 158, 127–137 (2002).
Clyne, P. J., Brotman, J. S., Sweeney, S. T. & Davis, G. Green fluorescent protein tagging Drosophila proteins at their native genomic loci with small P elements. Genetics 165, 1433–1441 (2003).
Kiehart, D., Galbraith, C., Edwards, K., Rickoll, W. & Montague, R. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149, 471–490 (2000).
Winter, C. G. et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105, 81–91 (2001).
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Sanson, B., White, P. & Vincent, J. P. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature 383, 627–630 (1996).
Franke, J., Montague, R. & Kiehart, D. Nonmuscle Myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Current Biology 15, 2208–2221 (2005).
Baker, N. E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 6, 1765–1773 (1987).
Young, P. E., Richman, A. M., Ketchum, A. S. & Kiehart, D. P. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 7, 29–41 (1993).
Tearle, R. G. & Nusslein-Volhard, C. Tübingen mutants and stock list. Dros. Inf. Serv. 66, 209–269 (1987).
Ryder, E. et al. The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics 177, 615–629 (2007).
Motzny, C. K. & Holmgren, R. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech. Dev. 52, 137–150 (1995).
Chandraratna, D., Lawrence, N., Welchman, D. P. & Sanson, B. An in vivo model of apoptosis: linking cell behaviours and caspase substrates in embryos lacking DIAP1. J. Cell Sci. 120, 2594–2608 (2007).
Kiehart, D. P. & Feghali, R. Cytoplasmic myosin from Drosophila melanogaster. J. Cell Biol. 103, 1517–1525 (1986).
Acknowledgements
We thank N. Lawrence for the initial observation of the segmental pattern of MyoII cables and for Supplementary Information, Movie 1; B. Bordbar for help in screening chromosomal deficiencies; D. Kiehart, R. Karess, G. Davis, J. Pradel, R. Holmgren, the UK Protein Trap Insertion Consortium, the Bloomington Stock Centre and the Developmental Studies Hybridoma Bank for reagents and B. Harris, R. Keynes, L. Perrin, M. Sémériva and D. St Johnston for critical reading of the manuscript. This work was funded by HFSP and Wellcome Trust grants to B.S; a Wellcome Trust Program Grant to A.H.B; an EMBO fellowship to A.P-M and ARC (Association pour la Recherche contre le Cancer) and Herchel Smith fellowships to B.M.
Author information
Authors and Affiliations
Contributions
Experimental work and data analysis were carried out by A.P-M and B.M. under supervision of A.H.B. and B.S. All authors contributed to data interpretation and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information Figures (PDF 1438 kb)
Supplementary Information
Supplementary Information Movie 1 (MOV 8051 kb)
Supplementary Information
Supplementary Information Movie 2 (MOV 6338 kb)
Supplementary Information
Supplementary Information Movie 3 (MOV 1104 kb)
Rights and permissions
About this article
Cite this article
Monier, B., Pélissier-Monier, A., Brand, A. et al. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol 12, 60–65 (2010). https://doi.org/10.1038/ncb2005
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2005
This article is cited by
-
Interlocking of co-opted developmental gene networks in Drosophila and the evolution of pre-adaptive novelty
Nature Communications (2023)
-
Self-assembly of tessellated tissue sheets by expansion and collision
Nature Communications (2022)
-
Differential cell adhesion implemented by Drosophila Toll corrects local distortions of the anterior-posterior compartment boundary
Nature Communications (2020)
-
A Cdc42-mediated supracellular network drives polarized forces and Drosophila egg chamber extension
Nature Communications (2020)
-
Morphogenesis of extra-embryonic tissues directs the remodelling of the mouse embryo at implantation
Nature Communications (2019)