Abstract
Invasive fungal pathogens are major causes of human mortality and morbidity1,2. Although numerous secreted effector proteins that reprogram innate immunity to promote virulence have been identified in pathogenic bacteria, so far, there are no examples of analogous secreted effector proteins produced by human fungal pathogens. Cryptococcus neoformans, the most common cause of fungal meningitis and a major pathogen in AIDS, induces a pathogenic type 2 response characterized by pulmonary eosinophilia and alternatively activated macrophages3,4,5,6,7,8. Here, we identify CPL1 as an effector protein secreted by C. neoformans that drives alternative activation (also known as M2 polarization) of macrophages to enable pulmonary infection in mice. We observed that CPL1-enhanced macrophage polarization requires Toll-like receptor 4, which is best known as a receptor for bacterial endotoxin but is also a poorly understood mediator of allergen-induced type 2 responses9,10,11,12. We show that this effect is caused by CPL1 itself and not by contaminating lipopolysaccharide. CPL1 is essential for virulence, drives polarization of interstitial macrophages in vivo, and requires type 2 cytokine signalling for its effect on infectivity. Notably, C. neoformans associates selectively with polarized interstitial macrophages during infection, suggesting a mechanism by which C. neoformans generates its own intracellular replication niche within the host. This work identifies a circuit whereby a secreted effector protein produced by a human fungal pathogen reprograms innate immunity, revealing an unexpected role for Toll-like receptor 4 in promoting the pathogenesis of infectious disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The primary read files as well as expression count files for RNA-seq data in this paper are available to download from the Gene Expression Omnibus under accession number GSE203483. Source data are provided with this paper.
References
Armstrong-James, D., Meintjes, G. & Brown, G. D. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol. 22, 120–127 (2014).
Brown, G. D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13 (2012).
Zhao, Y., Lin, J., Fan, Y. & Lin, X. Life cycle of Cryptococcus neoformans. Annu. Rev. Microbiol. 73, 17–42 (2019).
Müller, U. et al. Abrogation of IL-4 receptor-α-dependent alternatively activated macrophages is sufficient to confer resistance against pulmonary cryptococcosis despite an ongoing Th2 response. Int. Immunol. 25, 459–470 (2013).
Mueller, U. et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 179, 5367–5377 (2007).
Wiesner, D. L. et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 11, e1004701 (2015).
Schulze, B. et al. CD4+FoxP3+ regulatory T cells suppress fatal T helper 2 cell immunity during pulmonary fungal infection. Eur. J. Immunol. 44, 3596–3604 (2014).
Stenzel, W. et al. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am. J. Pathol. 174, 486–496 (2009).
Trompette, A. et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457, 585–588 (2009).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Eisenbarth, S. C. et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196, 1645–1651 (2002).
Millien, V. O. et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 341, 792–796 (2013).
Ademe, M. & Girma, F. Candida auris: from multidrug resistance to pan-resistant strains. Infect. Drug Resist. 13, 1287–1294 (2020).
Wall, G. & Lopez-Ribot, J. L. Current antimycotics, new prospects, and future approaches to antifungal therapy. Antibiotics 9, 445 (2020).
Selin, C., de Kievit, T. R., Belmonte, M. F. & Fernando, W. G. D. Elucidating the role of effectors in plant-fungal interactions: progress and challenges. Front. Microbiol. 7, 600 (2016).
Rajasingham, R. et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 17, 873–881 (2017).
Mueller, U. et al. Lack of IL-4 receptor expression on T helper cells reduces T helper 2 cell polyfunctionality and confers resistance in allergic bronchopulmonary mycosis. Mucosal Immunol. 5, 299–310 (2012).
Kindermann, M. et al. Group 2 innate lymphoid cells (ILC2) suppress beneficial type 1 immune responses during pulmonary cryptococcosis. Front. Immunol. 11, 209 (2020).
May, R. C., Stone, N. R. H., Wiesner, D. L., Bicanic, T. & Nielsen, K. Cryptococcus: from environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 14, 106–117 (2016).
Vecchiarelli, A. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 38, 407–417 (2000).
Liu, O. W. et al. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 135, 174–188 (2008).
Homer, C. M. et al. Intracellular action of a secreted peptide required for fungal virulence. Cell Host Microbe 19, 849–864 (2016).
Stergiopoulos, I. & de Wit, P. J. G. M. Fungal effector proteins. Annu. Rev. Phytopathol. 47, 233–263 (2009).
Arras, S. D. M., Chitty, J. L., Blake, K. L., Schulz, B. L. & Fraser, J. A. A genomic safe haven for mutant complementation in Cryptococcus neoformans. PLoS ONE 10, e0122916 (2015).
Brown, J. C. S. et al. Unraveling the biology of a fungal meningitis pathogen using chemical genetics. Cell 159, 1168–1187 (2014).
Kumar, P. et al. Pbx proteins in Cryptococcus neoformans cell wall remodeling and capsule assembly. Eukaryot. Cell 13, 560–571 (2014).
Kawakami, K., Zhang, T., Qureshi, M. H. & Saito, A. Cryptococcus neoformans inhibits nitric oxide production by murine peritoneal macrophages stimulated with interferon-gamma and lipopolysaccharide. Cell. Immunol. 180, 47–54 (1997).
Gibbs, K. D. et al. The Salmonella secreted effector SarA/SteE mimics cytokine receptor signaling to activate STAT3. Cell Host Microbe 27, 129–139.e4 (2020).
Panagi, I. et al. Salmonella effector SteE converts the mammalian serine/threonine kinase GSK3 into a tyrosine kinase to direct macrophage polarization. Cell Host Microbe 27, 41–53.e6 (2020).
Kasmi, El,K. C. et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 9, 1399–1406 (2008).
Deguine, J. & Barton, G. M. MyD88: a central player in innate immune signaling. F1000Prime Rep. 6, 97 (2014).
Lind, N. A., Rael, V., Pestal, K., Liu, B. & Barton, G. M. Regulation of the nucleic acid-sensing Toll-like receptors. Nat. Rev. Immunol. 22, 224–235 (2022).
Lancaster, G. I. et al. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 27, 1096–1110.e5 (2018).
Zanoni, I. et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147, 868–880 (2011).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Chevigné, A. & Jacquet, A. Emerging roles of the protease allergen Derp1 in house dust mite-induced airway inflammation. J. Allergy Clin. Immunol. 142, 398–400 (2018).
Jacquet, A. Characterization of innate immune responses to house dust mite allergens: pitfalls and limitations. Front. Allergy 2, 662378 (2021).
Evren, E., Ringqvist, E. & Willinger, T. Origin and ontogeny of lung macrophages: from mice to humans. Immunology 160, 126–138 (2020).
Makita, N., Hizukuri, Y., Yamashiro, K., Murakawa, M. & Hayashi, Y. IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int. Immunol. 27, 131–141 (2015).
Price, J. V. & Vance, R. E. The macrophage paradox. Immunity 41, 685–693 (2014).
Chun, C. D. & Madhani, H. D. Applying genetics and molecular biology to the study of the human pathogen Cryptococcus neoformans. Methods Enzymol. 470, 797–831 (2010).
Acknowledgements
We thank G. Barton for provision of Tlr2−/−, Tlr4−/− and Tlr2−/−Tlr4−/− mice, and R. Ricardo-Gonzalez for provision of Il4ra−/− and Stat6−/− mice; S. Chou for advice on protein purification; J. Cyster and E. Goldberg for critically reading the manuscript, discussions and advice; and S. Catania and M. Boucher for discussions and advice. Support was provided by the Chan–Zuckerberg Biohub, US National Institutes of Health, Jane Coffin Childs Memorial Fund for Medical Research Fellowship and Beckman Foundation.
Author information
Authors and Affiliations
Contributions
E.V.D. and H.D.M. conceived and designed the project. E.V.D. and H.D.M. designed experiments and wrote the manuscript. E.V.D. performed most of the experiments (including ELISA, flow cytometry and protein purification) and analysed the generated data. S.L. performed the forward genetic arrayed screen and helped with flow cytometry experiments. A.R. helped design and perform protein purification experiments. R.F.V. and B.W.Z. provided human monocyte-derived macrophages and read and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Gordon Brown and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1
(a) TNF ELISA on supernatants from BMDMs infected for 24 h with the indicated yeasts at MOI = 10. n = six biologically independent samples. Significance determined by one-way ANOVA with Bonferroni test. (b) Intracellular FACS staining for iNOS after 24 h of infection with either C. neoformans or S. cerevisiae at the indicated MOIs. n = three biologically independent samples. (c) RNA-seq heatmap depicting log2 fold changes of the indicated pro-inflammatory genes in BMDMs following 6 h of stimulation. (d) RNA-seq heatmap depicting log2 fold changes of the indicated M2/tolerized genes in BMDMs following 6 h of stimulation. (e) Representative FACS plots of ARG1 and iNOS expression in BMDMs following 24 h of stimulation with PBS, IL-4 (40 ng/ml), or LPS (100 ng/ml) and IFNγ (50 ng/ml). Data are presented as mean values +/– SD. ****p < 0.0001.
Extended Data Fig. 2
(a) Ranked Z-scores of hits from forward genetic screen for C. neoformans Arg1 induction. (b) List of validated screen hits and gene descriptions. (c) Representative FACS histograms of GXM staining on the indicated C. neoformans strains cultured overnight in 10% Sabouraud media. (d) RT-qPCR for CPL1 mRNA expression in cultures grown to OD600 = 1.0 in the indicated conditions (A.U. = arbitrary units relative to ACT1). n = three biologically independent samples. (e) Melanin production in WT or cpl1Δ strains grown at 30 °C on L-DOPA plates. (f) TNF production (measured by ELISA) to the indicated stimulations. n = six biologically independent samples. Significance determined by one-way ANOVA with Bonferroni test. Data are presented as mean values +/– SD. ****p < 0.0001.
Extended Data Fig. 3
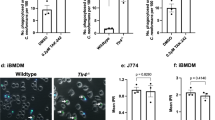
(a) Quantification by competitive ELISA of CPL1-6xHis in supernatants from the indicated strains grown in mammalian tissue culture conditions to OD = 1.0. n = six biologically independent samples. (b) RT-qPCR for Arg1 mRNA in BMDMs stimulated with the indicated C. neoformans strains (OD = 0.1) along with IL-4 (10 ng/ml) for 24 h. Expression normalized to Actb, a.u. = arbitrary units. n = three biologically independent samples. (c) RT-qPCR for Mrc1 mRNA in PMA-differentiated THP-1 cells stimulated with the indicated C. neoformans strains (OD = 0.1) along with IL-4 (10 ng/ml) for 24 h. Expression normalized to Actb, a.u. = arbitrary units. n = six biologically independent samples (d) RT-qPCR for Mrc1 mRNA in primary human monocyte-derived macrophages stimulated for 24 h with PBS or recombinant human IL-4 (10 ng/ml) along with the indicated C. neoformans strains (MOI = 0.1). Expression normalized to Actb, a.u. = arbitrary units. n = three biologically independent samples (e) RNA-seq read counts of the indicated genes in BMDMs stimulated for 24 h with either PBS, IL-4 (10 ng/ml), rCPL1 (111 nM), or IL-4 + rCPL1. n = three biologically independent samples. (f) Transwell migration assay on splenic eosinophils towards supernatants from BMDMs stimulated as in (e). n = three biologically independent samples. Data are presented as mean values +/– SD.
Extended Data Fig. 4
(a) Representative FACS staining of surface IL-4Rα levels on BMDMs stimulated for 24 h with the indicated C. neoformans strains (MOI = 10). n = six biologically independent samples (b) Phospho-FACS for pSTAT3 (left) and pSTAT6 (right) after 30 min of stimulation with PBS, IL-4 (10 ng/ml), rCPL1 (111 nM), or IL-4 + rCPL1. (c) Western blot for pSTAT6 or total STAT6 on BMDMs stimulated for the indicated times with either IL-4 (10 ng/ml) alone, rCPL1 (111 nM) alone, or IL-4 + rCPL1. Data are representative of three independent experiments. (d) Phospho-FACS for pSTAT3 (left) or pSTAT6 (right) in BMDMs after 8 h of stimulation with PBS, IL-4 (10 ng/ml), rCPL1 (37 nM), or rCPL1+IL-4. (e) Phospho-FACS for pSTAT3 in BMDMs stimulated with 111 nM rCPL1 for the indicated time points. Data are presented as mean values +/– SD.
Extended Data Fig. 5
(a) Arginase-1 FACS in Myd88+/+ or Myd88–/– BMDMs stimulated for 24 h with the indicated concentrations of rCPL1 alone (left) or in combination with IL-4 (10 ng/ml). n = three biologically independent samples. (b) Arginase-1 FACS gated on CD45.2+ BMDMs from the indicated genotypes co-cultured with a 50:50 mix of CD45.1 BoyJ BMDMs and stimulated for 24 h with IL-4 (10 ng/ml) or IL-4 + rCPL1 (111 nM). n = three biologically independent samples. (c) Arginase-1 FACS on BMDMs stimulated for 24 h with the indicated concentrations of LPS. (d) Measurement of pyroptosis by LDH release assay on BMDMs stimulated with the indicated concentrations of LPS alone or with 10 ug/ml cholera toxin B (CTB). n = three biologically independent samples. (e) Measurement of pyroptosis by LDH release assay on BMDMs stimulated with the indicated concentrations of rCPL1 alone or with 10 ug/ml CTB. n = three biologically independent samples. (f) Arginase-1 FACS on BMDMs stimulated with rCPL1 that was either kept on ice or boiled at 100 °C for 15 min. Cells were stimulated with either rCPL1 alone (left) or in combination with IL-4 (right). n = three biologically independent samples. (g)(h) Arginase-1 FACS in in BMDMs stimulated with the indicated concentrations of rCPL1 alone (g) or in combination with IL-4 (10 ng/ml) (h) that were either treated with control or polymyxinB. n = three biologically independent samples. (i) Arginase-1 FACS on BMDMs transduced with MSCV-empty or MSCV-CPL1 retrovirus and stimulated for 24 h with the indicated concentrations of IL-4. n = three biologically independent samples. (j) Silver stain on SDS-PAGE gel of rCPL1-6xHis or rCPL1(Y160A)-6xHis purified from P. pastoris. Image is representative of three independent experiments. Data are presented as mean values +/– SD. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 by one-way ANOVA with Bonferroni test.
Extended Data Fig. 6
(a) Arginase-1 FACS in Tlr4+/+ or Tlr4–/– BMDMs stimulated for 24 h with the indicated concentrations of rCPL1-6xHis purified from C.n. supernatants alone or in combination with IL-4 (10 ng/ml) (b). n = three biologically independent samples. Data are presented as mean values +/– SD. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 by one-way ANOVA with Bonferroni test.
Extended Data Fig. 7
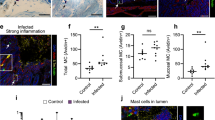
(a) Representative FACS gating and quantification of lung eosinophils after 10 days of intranasal infection with the indicated C.n. strains. (b) Representative FACS gating and quantification of mediastinal lymph node GC B cells. (c) Representative FACS gating and quantification of GC B cell antibody isotype. (d) Representative FACS gating and quantification of effector CD4+ T cells. (e) Representative FACS gating and quantification of cytokine production from effector CD4+ T cells after 4hrs of stimulation with PMA, Ionomycin, and GolgiSTOP. **p < 0.01 by one-way ANOVA with Bonferroni test.
Extended Data Fig. 8
(a) Quantification of YARG expression by FACS in lung interstitial macrophages in mice infected for 10 days with 5x104 CFU of the indicated strains. (b) Quantification of eosinophils in mice infected for 10 days with 5x104 CFU of the indicated strains. (c) Kaplan-Meier survival curve analysis of mice infected with WT, cpl1Δ, or cpl1Δ+CPL1 C.n. (N = 6 mice per group); ****p < 0.0001 by Mantel-Cox test. (d) Brain CFUs from mice infected with WT, cpl1Δ, or cpl1Δ+CPL1 C.n (5x104 CFU) for 14 days. (e) Lung CFUs on G418-non-resistant (left) or -resistant (right) colonies from mice infected for 10 days with a 50:50 mix of the indicated strains.
Extended Data Fig. 9
(a) Quantification of lung eosinophils in WT or Tlr4–/– mice infected for 10 days with 5x104 CFU C. neoformans. (b) Lung CFUs from Tlr4–/– or Tlr4–/– mice infected with wild type C.n. (5x104 CFU) for 10 days (c) FACS quantification of lung eosinophils in WT (N = 6 mice) or Tlr4–/– (N = 4 mice) mice sensitized intranasally with rCPL1. (d) Model of how secreted CPL1 modulates the macrophage inflammatory state (created using Biorender.com). p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 by one-way ANOVA with Bonferroni test.
Supplementary information
Supplementary Figure 1
Uncropped gel images from western blots for pSTAT3 and pSTAT6.
Source data
Rights and permissions
About this article
Cite this article
Dang, E.V., Lei, S., Radkov, A. et al. Secreted fungal virulence effector triggers allergic inflammation via TLR4. Nature 608, 161–167 (2022). https://doi.org/10.1038/s41586-022-05005-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05005-4
This article is cited by
-
Fungus hijacks TLR4 to build a type 2 immune niche
Nature Reviews Immunology (2022)
-
Host and Environmental Sensing by Entomopathogenic Fungi to Infect Hosts
Current Clinical Microbiology Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.