Abstract
Mutations of the tricarboxylic acid cycle enzyme fumarate hydratase cause hereditary leiomyomatosis and renal cell cancer1. Fumarate hydratase-deficient renal cancers are highly aggressive and metastasize even when small, leading to a very poor clinical outcome2. Fumarate, a small molecule metabolite that accumulates in fumarate hydratase-deficient cells, plays a key role in cell transformation, making it a bona fide oncometabolite3. Fumarate has been shown to inhibit α-ketoglutarate-dependent dioxygenases that are involved in DNA and histone demethylation4,5. However, the link between fumarate accumulation, epigenetic changes, and tumorigenesis is unclear. Here we show that loss of fumarate hydratase and the subsequent accumulation of fumarate in mouse and human cells elicits an epithelial-to-mesenchymal-transition (EMT), a phenotypic switch associated with cancer initiation, invasion, and metastasis6. We demonstrate that fumarate inhibits Tet-mediated demethylation of a regulatory region of the antimetastatic miRNA cluster6 mir-200ba429, leading to the expression of EMT-related transcription factors and enhanced migratory properties. These epigenetic and phenotypic changes are recapitulated by the incubation of fumarate hydratase-proficient cells with cell-permeable fumarate. Loss of fumarate hydratase is associated with suppression of miR-200 and the EMT signature in renal cancer and is associated with poor clinical outcome. These results imply that loss of fumarate hydratase and fumarate accumulation contribute to the aggressive features of fumarate hydratase-deficient tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
ArrayExpress
Gene Expression Omnibus
Data deposits
RNA-seq data are deposited at the Gene Expression Omnibus under accession number GSE77542 and gene expression data for Sdhb-deficient cells are deposited at ArrayExpress under accession number E-MTAB-4349.
Change history
19 October 2016
A Correction to this paper has been published: https://doi.org/10.1038/nature20144
References
Tomlinson, I. P. et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 30, 406–410 (2002)
Schmidt, L. S. & Linehan, W. M. Hereditary leiomyomatosis and renal cell carcinoma. Int. J. Nephrol. Renovasc. Dis. 7, 253–260 (2014)
Yang, M., Soga, T., Pollard, P. J. & Adam, J. The emerging role of fumarate as an oncometabolite. Front Oncol. 2, 85 (2012)
Laukka, T. et al. Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. J. Biol. Chem. 291, 4256–4265 (2016)
Xiao, M. et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 26, 1326–1338 (2012)
De Craene, B. & Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 13, 97–110 (2013)
Frezza, C. et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature 477, 225–228 (2011)
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
Puisieux, A., Brabletz, T. & Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 16, 488–494 (2014)
Yang, M.-H. et al. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat. Cell Biol. 10, 295–305 (2008)
Isaacs, J. S. et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8, 143–153 (2005)
Bertout, J. A., Patel, S. A. & Simon, M. C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 8, 967–975 (2008)
Davalos, V. et al. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene 31, 2062–2074 (2012)
Song, S. J. et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 154, 311–324 (2013)
Hu, X. et al. Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell reprogramming. Cell Stem Cell 14, 512–522 (2014)
Heinemann, B. et al. Inhibition of demethylases by GSK-J1/J4. Nature 514, E1–E2 (2014)
Karolchik, D. et al. The UCSC Genome Browser database: 2014 update. Nucleic Acids Res. 42, D764–D770 (2014)
Hagège, H. et al. Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat. Protocols 2, 1722–1733 (2007)
MacKenzie, E. D. et al. Cell-permeating α-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol. Cell. Biol. 27, 3282–3289 (2007)
Buchmaier, B. S. et al. Renal cells express different forms of vimentin: the independent expression alteration of these forms is important in cell resistance to osmotic stress and apoptosis. PLoS One 8, e68301 (2013)
Zheng, L. et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat. Commun. 6, 6001 (2015)
Sullivan, L. B. et al. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol. Cell 51, 236–248 (2013)
Cardaci, S. et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat. Cell Biol. 17, 1317–1326 (2015)
Letouzé, E. et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 23, 739–752 (2013)
Ooi, A. et al. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell 20, 511–523 (2011)
Linehan, W. M. et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N. Engl. J. Med. 374, 135–145 (2016)
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013)
Fang, Y. et al. Protein expression of ZEB2 in renal cell carcinoma and its prognostic significance in patient survival. PLoS One 8, e62558 (2013)
Fieuw, A. et al. Identification of a novel recurrent 1q42.2-1qter deletion in high risk MYCN single copy 11q deleted neuroblastomas. Int. J. Cancer 130, 2599–2606 (2012)
Ashrafian, H. et al. Expression profiling in progressive stages of fumarate-hydratase deficiency: the contribution of metabolic changes to tumorigenesis. Cancer Res. 70, 9153–9165 (2010)
Li, L. C. & Dahiya, R. MethPrimer: designing primers for methylation PCRs. Bioinformatics 18, 1427–1431 (2002)
Piccinini, F., Kiss, A. & Horvath, P. CellTracker (not only) for dummies. Bioinformatics 32, 955–957 (2016)
Schmidt, D. et al. ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods 48, 240–248 (2009)
Rajeeve, V., Vendrell, I., Wilkes, E., Torbett, N. & Cutillas, P. R. Cross-species proteomics reveals specific modulation of signaling in cancer and stromal cells by phosphoinositide 3-kinase (PI3K) inhibitors. Mol. Cell. Proteomics 13, 1457–1470 (2014)
Casado, P. et al. Kinase-substrate enrichment analysis provides insights into the heterogeneity of signaling pathway activation in leukemia cells. Sci. Signal. 6, rs6 (2013)
Casado, P. & Cutillas, P. R. A self-validating quantitative mass spectrometry method for assessing the accuracy of high-content phosphoproteomic experiments. Mol. Cell. Proteomics 10, M110003079 (2011)
Cutillas, P. R. & Vanhaesebroeck, B. Quantitative profile of five murine core proteomes using label-free functional proteomics. Mol. Cell. Proteomics 6, 1560–1573 (2007)
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015)
Taube, J. H. et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc. Natl Acad. Sci. USA 107, 15449–15454 (2010)
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014)
Acknowledgements
This work was supported by the Medical Research Council (UK). S.F. was supported by a Herchel Smith Research Studentship and K.F. by an MRC Career Development Award. E.R.M is supported by the ERC Advanced Researcher award 323004–ONCOTREAT. P.H.M. is supported by Senior Investigator Awards from the Wellcome Trust and NIHR. The Cambridge Human Research Tissue Bank and A.W. are supported by the NIHR Cambridge Biomedical Research Centre. We thank the UOB Tumor Cell Line Repository and W. Linehan for UOK262 cell lines. We thank E. Clemente, J. Bauer, and the staff at Cambridge Genomic Services for miRNA profiling; the CRUK Cambridge Institute (Genomics facility), especially J. Hadfield, and the CRUK Cambridge Institute (Bioinformatics facility), especially C. S. R. Chilamakuri for RNA-seq sample processing and analysis. We also thank C. Martins and A. Rasola for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
M.S. and C.F. conceived the study. M.S. performed and analysed all the experiments on cell lines with the help of A.V.D., S.R.A. and S.J.T., and prepared the figures. E.Gon. performed the bioinformatics analyses with the supervision of J.S.-R. T.I.J. helped M.S. with the invasion assays and generation of constructs for miRNA and Fh1–GFP expression. V.R.Z. performed and analysed ChIP–PCR assays. A.S.H.d.C. performed and analysed all the metabolomics analyses with the help of E.Ga. M.G.B.T. performed the work on human samples with input from P.H.M. A.W., V.G., P.H.M. and E.R.M. provided the HLRCC samples. V.R and P.C. performed the proteomics analyses. H.Y. and B.H. supervised and performed the 3C experiments. S.C. and E.Gott. provided Sdhb-deficient cells and generated the gene expression profiles of these cells. S.F. and K.F. performed cell motility assays. C.F. directed the research, prepared the figures and wrote the paper, with assistance from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks G. Goodall and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Characterization of Fh1-deficient and Fh1-rescued cells.
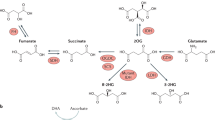
a, PCR to assess Fh1 recombination. Putative genotypes are indicated on the right and are based on the expected size of the genomic PCR amplification products as from ref. 7. Fh1fl/fl = 470 bp and Fh1−/− = 380 bp. b, Fh1 protein levels measured by western blotting of cells of the indicated genotype. Calnexin was used as loading control. c, Intracellular fumarate levels measured by LC–MS and normalized to total ion count. Results were obtained from four independent cultures and are indicated as average ± s.d. P values were calculated from one-way ANOVA. d, OCR and ECAR assessed using the Seahorse Extracellular Flux Analyser. Results were obtained from five replicate wells and are presented as average ± s.d. e, Bright-field images of cells of the indicated phenotype. Scale bar, 400 μm. Western blot and gel source images are presented in Supplementary Fig. 1. Source data are presented in Supplementary Table 2. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. f, Schematic representation of the proposed link between loss of FH, fumarate accumulation, and epigenetic suppression of the antimetastatic cluster of miRNA miR-200. Upon accumulation of fumarate as a result of FH inactivation, the TET-mediated demethylation of the mir-200ba429 cluster is inhibited, leading to their epigenetic suppression. As a consequence, Zeb1 and Zeb2 are de-repressed, eliciting a signalling cascade that leads to EMT.
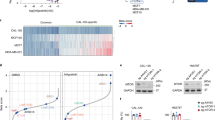
Extended Data Figure 2 EMT signature in Fh1−/− cells.
a, Volcano plots of RNA-seq analysis. Gene expression was normalized to Fh1fl/fl or Fh1−/−+pFh1 cells as indicated. b, c, GSEA (b) and EMT enrichment score (c) of the indicated cell lines.
Extended Data Figure 3 EMT signature in UOK262 cells.
a, GSEA and EMT enrichment score of the indicated cell lines. Gene expression was normalized to UOK262pFH cells. b, c, mRNA expression measured by qPCR (b) and protein levels measured by western blot (c) of the indicated EMT markers. d, Immunofluorescence staining for vimentin and E-cadherin. DAPI was used as marker for cell nuclei. Scale bar, 25 μm. e, Cell migration rate. Results were obtained from 14 replicate wells and presented as mean ± s.d. f, mRNA expression of EMT-related transcription factors ZEB1 and ZEB2 from RNA-seq data as in Fig. 1a. g, Expression levels of the indicated miRNAs measured by qPCR. h, Volcano plot of miRNA profiling. All qPCR data were obtained from three independent experiments and presented as RQ with maximum values, normalized to endogenous controls ACTB for mRNA, or RNU6-6P and SNORD61 for miRNA analyses. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Western blot source images are presented in Supplementary Fig. 1. Source data are presented in Supplementary Table 2.
Extended Data Figure 4 EMT features in Fh1-deficient cells are independent of HIF.
mRNA levels of EMT genes and Hif1β (a) and HIF target genes (b) in Fh1−/− cells infected with shRNA against Hif1β measured by qPCR. Results were obtained from three independent cultures and presented as RQ with maximum values using Actb as endogenous control. P values from unpaired t-test are indicated. Glut1 also known as Slc2a1. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Source data are presented in Supplementary Table 2.
Extended Data Figure 5 EMT signature in Fh1-reconstituted cells.
a, Fh1 protein levels measured by western blot. Calnexin was used as loading control. b, Intracellular fumarate levels measured by LC–MS. Data are obtained from five independent cultures and presented as average ± s.d. c, Representative bright-field images of cells of the indicated genotypes. Scale bar, 400 μm. d, e, mRNA expression measured by qPCR (d) and protein levels measured by western blot (e) of the indicated EMT markers. f, Average speed of cells calculated after tracking cells for 3 h as in Fig. 1g. Results were generated from three independent cultures. g, mRNA expression of EMT-related transcription factors. Actb was used as endogenous control. EV, empty vector. h, Expression levels of the indicated miRNAs measured by qPCR and normalized to Snord95 and Snord61 as endogenous controls. All qPCR results were obtained from three independent cultures and presented as RQ with maximum values. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Western blot source images are presented in Supplementary Fig. 1. Source data are presented in Supplementary Table 2.
Extended Data Figure 6 Role of Tets and histone demethylases in EMT induction.
a, Expression levels of Tet1, Tet2 and Tet3 in Fh1fl/fl cells from RNA-seq data. b–d, Expression levels of Tet2 and Tet3 (b), miR-200 (c), and Cdh1 (E-cadherin) (d) in Fh1fl/fl cells upon combined silencing of Tet2 and Tet3. The results are presented as RQ with maximum values obtained from technical replicates. Actb and Snord61 were used as endogenous controls for mRNA and miRNA, respectively. e, Expression levels of the indicated miRNAs upon inhibition of histone demethylases by GSK-J4. Snord61 and Snord95 were used as endogenous controls. f, Expression of the indicated miRNAs in Fh1−/− cells incubated for 24 h with 5 mM DM-aKG measured by qPCR. Results were obtained from four (vehicle), five (Fh1−/−CL19 DM-aKG) or three (Fh1−/−CL1 DM-aKG) independent cultures and presented as RQ with maximum values, normalized to Snord95 as endogenous control. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Extended Data Figure 7 Characterization of the regulatory CpG island CpG43.
a, Snapshot of Genome Browser view of genomic DNA around the mir-200ba429 cluster taken from NCBI37/mm9. Tet2 ChIP was obtained from GSE41720, sample GSM1023124. Shaded rectangles indicate mir-200ba429 and CpG43. b, ChIP–PCR of the indicated histone marks in a region adjacent CpG43. Data were obtained from three independent cultures and are presented as average ± s.d. P values from unpaired t-tests are indicated. c, Expression levels of H3 histone marks in cells of the indicated genotypes measured by western blot with H3 and calnexin as loading controls. d, 3C data for the genomic region adjacent to CpG43 analysed in Fh1fl/fl cells. The positions of CpG30 and CpG43 and of the predicted restriction sites are indicated. Results were generated from two independent cultures. e, DNA methylation of CpG43 assessed by qPCR using OneStep qMethyl kit. Data were obtained from three independent experiments and normalized to methylation levels of the region in Fh1fl/fl cells. Data are presented as average ± s.e.m. f, ChIP–PCR of Tet binding to CpG43. Data were obtained from three replicates and are presented as average ± s.d. g, 5hmC nuclear staining assessed by immunofluorescence using 5hmC antibody. Nuclear staining was quantified using Image J and an average of 120 cells was used per genotype. P values from one-way ANOVA. Representative images of 5hmC staining are shown. DAPI is used to stain nuclei. Scale bar, 20 μm. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Western blot source images are presented in Supplementary Fig. 1. Source data are presented in Supplementary Table 2.
Extended Data Figure 8 MMF triggers EMT in FH-proficient cells.
a, Bright-field images of cells treated for 6 weeks with MMF. Arrows indicate the typical protrusion of cells of mesenchymal phenotype. Scale bar, 400 μm. b, OCR of the indicated cell lines treated chronically with MMF (as in Fig. 3). See Methods for drug concentrations. OCR was normalized to total protein content. Results were obtained from six (for mouse cells) or eight (for human cells) wells, data are shown ± s.d. c, Hive plot of metabolomics data of mouse and human cells treated with MMF (as in Fig. 3). All identified metabolites are included on the y-axis and grouped into human (pink) and mouse (green) cells. Metabolites accumulated (right x-axis) or depleted (left x-axis) in MMF-treated cells versus controls are indicated by a connecting arc and their fold-change is colour-coded. Metabolites accumulated commonly across the two cell lines are highlighted with a solid line. 2SC, 2-succinic-cysteine; succGSH, succinic-GSH. Source data are presented in Supplementary Table 2. Source metabolomic data are presented in Supplementary Table 3.
Extended Data Figure 9 Succinate triggers EMT in Sdhb-deficient cells.
a, Intracellular succinate levels after incubation with 4 mM MMS measured by LC–MS. Data are presented as average ± s.d. b, c, Intracellular succinate (b) and succGSH (c) levels in Sdhb-deficient cells measured by LC–MS. Data are presented as average ± s.d. d, Bright-field images of cells of the indicated genotype. Scale bar, 400 μm. e, GSEA and EMT enrichment score from expression analysis of the indicated cell lines. f, g, miRNA expression levels normalized to Snord61 and Snord95 as endogenous controls (f) and CpG43 methylation (g). Experiments were performed as in Fig. 2b and d, respectively. Metabolomics experiments were obtained from five independent cultures. qPCR results were generated from three independent cultures. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Gel source images are presented in Supplementary Fig. 1. Source data are presented in Supplementary Table 2.
Extended Data Figure 10 Expression of FH and EMT markers in kidney cancer.
a, Expression levels of VIM and CDH1 in HLRCC samples obtained from ref. 25. b, Immunohistochemistry staining for vimentin and E-cadherin (left) and TET1 and TET2 (right) in samples from HLRCC patients obtained as in Fig. 4a. Scale bar, 100 μm. Inset in left panel indicates a 3× digital magnification. Scale bar, 50 μm. c, GSEA and EMT enrichment score from RNA-seq data for KIRP from ref. 26. d, Volcano plot of miRNA expression in KIRP. e, Kaplan–Meier survival curve for KIRP patients separated according to FH expression. f, VIM and CDH1 expression in FH-mutant KIRP samples compared to normal renal tissue. g, Frequency of mutations in FH and TET1, TET2 and TET3 in KIRP samples analysed using NCBO BioPortal. Only cancers with mutations in the indicated genes are shown. h, Kaplan–Meier curve for KIRP samples containing wild-type or mutant FH. i, Expression levels of FH, VIM and CDH1 in KIRC samples obtained from TCGA data set27. j, Volcano plot of miRNA expression in KIRC samples. j, Kaplan–Meier curve of patients with KIRC separated according to FH expression.
Supplementary information
Supplementary Information
This file contains Supplementary Figure 1, which includes western blot and gel Source Data. (PDF 683 kb)
Supplementary Tables
This zipped file contains Supplementary Tables 1-4. (ZIP 559 kb)
Source data
Rights and permissions
About this article
Cite this article
Sciacovelli, M., Gonçalves, E., Johnson, T. et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 537, 544–547 (2016). https://doi.org/10.1038/nature19353
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19353
This article is cited by
-
A break in mitochondrial endosymbiosis as a basis for inflammatory diseases
Nature (2024)
-
Metabolic alterations in hereditary and sporadic renal cell carcinoma
Nature Reviews Nephrology (2024)
-
Identifying new biomarkers of aggressive Group 3 and SHH medulloblastoma using 3D hydrogel models, single cell RNA sequencing and 3D OrbiSIMS imaging
Acta Neuropathologica Communications (2023)
-
Retrograde regulation of mitochondrial fission and epithelial to mesenchymal transition in hepatocellular carcinoma by GCN5L1
Oncogene (2023)
-
Genomic and transcriptomic features between primary and paired metastatic fumarate hydratase–deficient renal cell carcinoma
Genome Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.