Abstract
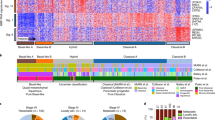
Integrated genomic analysis of 456 pancreatic ductal adenocarcinomas identified 32 recurrently mutated genes that aggregate into 10 pathways: KRAS, TGF-β, WNT, NOTCH, ROBO/SLIT signalling, G1/S transition, SWI-SNF, chromatin modification, DNA repair and RNA processing. Expression analysis defined 4 subtypes: (1) squamous; (2) pancreatic progenitor; (3) immunogenic; and (4) aberrantly differentiated endocrine exocrine (ADEX) that correlate with histopathological characteristics. Squamous tumours are enriched for TP53 and KDM6A mutations, upregulation of the TP63∆N transcriptional network, hypermethylation of pancreatic endodermal cell-fate determining genes and have a poor prognosis. Pancreatic progenitor tumours preferentially express genes involved in early pancreatic development (FOXA2/3, PDX1 and MNX1). ADEX tumours displayed upregulation of genes that regulate networks involved in KRAS activation, exocrine (NR5A2 and RBPJL), and endocrine differentiation (NEUROD1 and NKX2-2). Immunogenic tumours contained upregulated immune networks including pathways involved in acquired immune suppression. These data infer differences in the molecular evolution of pancreatic cancer subtypes and identify opportunities for therapeutic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
ArrayExpress
Gene Expression Omnibus
Data deposits
All DNA sequencing and RNA-seq data have been deposited in the European Genome-phenome Archive (EGA): accession code EGAS00001000154. All gene expression, genotyping, and methylome data used in this study has been deposited in the NCBI Gene Expression Omnibus (GEO) under accession codes GSE49149 and GSE36924. Mouse cell line expression data are available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-4415.
Change history
02 March 2016
A present address was added for author R.G.
References
Rahib, L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014)
Waddell, N. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501 (2015)
Jones, S. et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, 1801–1806 (2008)
Wang, L. et al. Whole-exome sequencing of human pancreatic cancers and characterization of genomic instability caused by MLH1 haploinsufficiency and complete deficiency. Genome Res. 22, 208–219 (2012)
Biankin, A. V. et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491, 399–405 (2012)
Alexandrov, L. B., Nik-Zainal, S., Wedge, D. C., Campbell, P. J. & Stratton, M. R. Deciphering signatures of mutational processes operative in human cancer. Cell Rep . 3, 246–259 (2013)
Nones, K. et al. Genome-wide DNA methylation patterns in pancreatic ductal adenocarcinoma reveal epigenetic deregulation of SLIT-ROBO, ITGA2 and MET signaling. Int. J. Cancer 135, 1110–1118 (2014)
Nones, K. et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nature Commun . 5, 5224 (2014)
Patch, A. M. et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 521, 489–494 (2015)
The Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550 (2014)
Bechara, E. G., Sebestyen, E., Bernardis, I., Eyras, E. & Valcarcel, J. RBM5, 6, and 10 differentially regulate NUMB alternative splicing to control cancer cell proliferation. Mol. Cell 52, 720–733 (2013)
Scott, L. M. & Rebel, V. I. Acquired mutations that affect pre-mRNA splicing in hematologic malignancies and solid tumors. J. Natl. Cancer Inst. 105, 1540–1549 (2013)
Maguire, S. L. et al. SF3B1 mutations constitute a novel therapeutic target in breast cancer. J. Pathol. 235, 571–580 (2015)
Horn, S. et al. Mind bomb 1 is required for pancreatic β-cell formation. Proc. Natl Acad. Sci. USA 109, 7356–7361 (2012)
Scaltriti, M. et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc. Natl Acad. Sci. USA 108, 3761–3766 (2011)
Shain, A. H., Salari, K., Giacomini, C. P. & Pollack, J. R. Integrative genomic and functional profiling of the pancreatic cancer genome. BMC Genomics 14, 624 (2013)
Tubio, J. M. et al. Mobile DNA in cancer. Extensive transduction of nonrepetitive DNA mediated by L1 retrotransposition in cancer genomes. Science 345, 1251343 (2014)
Hoadley, K. A. et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 158, 929–944 (2014)
Brody, J. R. et al. Adenosquamous carcinoma of the pancreas harbors KRAS2, DPC4 and TP53 molecular alterations similar to pancreatic ductal adenocarcinoma. Mod. Pathol. 22, 651–659 (2009)
Engelmann, D. & Putzer, B. M. Emerging from the shade of p53 mutants: N-terminally truncated variants of the p53 family in EMT signaling and cancer progression. Sci. Signal. 7, re9 (2014)
Hale, M. A. et al. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Dev. Biol. 286, 225–237 (2005)
von Figura, G., Morris, J. P. IV, Wright, C. V. & Hebrok, M. Nr5a2 maintains acinar cell differentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiation. Gut 63, 656–664 (2014)
Hale, M. A. et al. The nuclear hormone receptor family member NR5A2 controls aspects of multipotent progenitor cell formation and acinar differentiation during pancreatic organogenesis. Development 141, 3123–3133 (2014)
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G. & Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015)
Weissmueller, S. et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling. Cell 157, 382–394 (2014)
Miller, B. W. et al. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol. Med. 7, 1063–1076 (2015)
Collisson, E. A. et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature Med. 17, 500–503 (2011)
Moffitt, R. A. et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nature Genet. 47, 1168–1178 (2015)
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov . 2, 401–404 (2012)
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013)
Gonzalez-Perez, A. et al. IntOGen-mutations identifies cancer drivers across tumor types. Nature Methods 10, 1081–1082 (2013)
Leiserson, M. D. et al. Pan-cancer network analysis identifies combinations of rare somatic mutations across pathways and protein complexes. Nature Genet. 47, 106–114 (2015)
Razick, S., Magklaras, G. & Donaldson, I. M. iRefIndex: a consolidated protein interaction database with provenance. BMC Bioinformatics 9, 405 (2008)
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011)
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010)
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013)
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014)
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014)
Leek, J. T., Johnson, W. E., Parker, H. S., Jaffe, A. E. & Storey, J. D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28, 882–883 (2012)
Kuehn, H., Liberzon, A., Reich, M. & Mesirov, J. P. Using GenePattern for gene expression analysis. Current Protoc. Bioinformatics Chapter 7, Unit 7 12, (2008)
Wilkerson, M. D. & Hayes, D. N. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics 26, 1572–1573 (2010)
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014)
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14, 7 (2013)
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008)
Langfelder, P., Zhang, B. & Horvath, S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 24, 719–720 (2008)
Fang, H. & Gough, J. The ‘dnet’ approach promotes emerging research on cancer patient survival. Genome Med . 6, 64 (2014)
Wu, G. & Stein, L. A network module-based method for identifying cancer prognostic signatures. Genome Biol. 13, R112 (2012)
Du, P., Kibbe, W. A. & Lin, S. M. lumi: a pipeline for processing Illumina microarray. Bioinformatics 24, 1547–1548 (2008)
Tan, E. H. et al . Functions of TAp63 and p53 in restraining the development of metastatic cancer. Oncogene 25, 3325–3333 (2014)
Yoshihara, K. et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nature Commun . 4, 2612 (2013)
Acknowledgements
We would like to thank C. Axford, M.-A. Brancato, S. Rowe, M. Thomas, S. Simpson and G. Hammond for central coordination of the Australian Pancreatic Cancer Genome Initiative, data management and quality control; M. Martyn-Smith, L. Braatvedt, H. Tang, V. Papangelis and M. Beilin for biospecimen acquisition; and Deborah Gwynne for support at the Queensland Centre for Medical Genomics. We also thank M. Hodgins, M. Debeljak and D. Trusty for technical assistance at Johns Hopkins University. Funding support was from: National Health and Medical Research Council of Australia (NHMRC; 631701, 535903, 427601); Queensland Government (NIRAP); University of Queensland; Australian Government: Department of Innovation, Industry, Science and Research (DIISR); Australian Cancer Research Foundation (ACRF); Cancer Council NSW: (SRP06-01, SRP11-01. ICGC); Cancer Institute NSW: (10/ECF/2-26; 06/ECF/1-24; 09/CDF/2-40; 07/CDF/1-03; 10/CRF/1-01, 08/RSA/1-15, 07/CDF/1-28, 10/CDF/2-26,10/FRL/2-03, 06/RSA/1-05, 09/RIG/1-02, 10/TPG/1-04, 11/REG/1-10, 11/CDF/3-26); Garvan Institute of Medical Research; Cancer Research UK Glasgow Centre Program, A18076; Avner Nahmani Pancreatic Cancer Research Foundation; R.T. Hall Trust; Petre Foundation; Philip Hemstritch Foundation; Gastroenterological Society of Australia (GESA); American Association for Cancer Research (AACR) Landon Foundation—INNOVATOR Award; Wellcome Trust Senior Investigator Award 103721/Z/14/Z; Cancer Research UK Programme Grant C29717/A17263; Cancer Research UK Programme Grant A12481; Pancreatic Cancer UK; The Howat Foundation; University of Glasgow; European Research Council Starting Grant, 311301, Italian Ministry of University and Research (Cancer Genome Project FIRB RBAP10AHJB), Associazione Italiana Ricerca Cancro (n.12182) , Fondazione Italiana Malattie Pancreas – Ministry of Health (CUP_J33G13000210001), European Community Grant FP7 Cam-Pac, grant agreement number 602783.
Author information
Authors and Affiliations
Consortia
Contributions
Investigator contributions are as follows: P.J.B., J.V.P., N.W., A.V.B., S.M.G. (concept and design); P.J.B., D.A.W., R.A.G., A.S., D.K.C., J.V.P., N.W., A.V.B., S.M.G. (project leaders); P.J.B., D.K.C., A.V.B., S.M.G. (writing team); D.K.M., A.N.C., T.J.C.B., C.N., K.N., S.W., D.M.M., N.W., L.E., L.M., L.S., S.M.G., I.H., S.I., S.M., E.N., K.Q., S.M.G. (genomics); P.J.B., D.K.M., K.S.K., N.W., P.J.W., O. H., A.M.P., F.N., O.H., C.L., D.T., S.W., Q.X., K.N., N.C., M.Q., M.A., A.R., M.G., S.K., K.Q., L.P., J.M., M.C., L.C.M., O.S., L.F., U.B., N.W., J.V.P., S.M.G. (data analysis); D.K.C., A.L.J., A.M.N., A.M., A.V.P., C.W.T., E.K.C., E.S.H., I.R., M.G., J.H., J.A.L., K.E., L.A.C., M.D.J., A.J.G., N.Q.N., A.B., N.Z., C.P., R.G., J.R.E., R.H.H., A.M., C.A.I., C.L.W., B.R., V.C., P.C., C.B., R.S., G.T., D.M., G.M.P., J.H., M.P., J.W., V.C., C.J.S., J.G.K., R.T.L., N.D.M., N.B.J., J.S.G., J.D.S., R.A.M., J.H., S.A.K., K.M., R.L.S., A.V.B. (sample acquisition and processing, clinical annotation, interpretation and analysis); A.J.G., A.C., R.H.H., F.D., K.O., A.S., W.F., J.G.K., C.T. (pathology assessment).
Corresponding authors
Ethics declarations
Competing interests
R.H.H. receives royalty payments from Myriad Genetics for the PALB2 invention.
Additional information
A list of authors and affiliations appears in the Supplementary Information.
Extended data figures and tables
Extended Data Figure 1 Mutational landscape of PC.
a, Barplot representing the somatic mutation rate for each of the 456 samples included in this analysis.b, Non-silent mutations (blue), amplifications (≥8 copies, red), deletions (purple) and structural variants (SV, green) ranked in order of exclusivity. c, Significantly mutated genes identified by OncodriverFM. An asterisk denotes a significantly mutated gene identified by both MutSigCV and OncodriverFM. d, PC mutation functional interaction (FI) sub-network identified by the ReactomeFI cytoscape plugin. Mutated genes are indicated as coloured circles and linker genes (that is, genes not significantly mutated but highly connected to mutated genes in the network) indicated as coloured diamonds. Different node colours indicate different network clusters or closely interconnected genes. P values represent FDR < 0.05. Pathways significantly enriched in the identified FI sub-network are shown in the accompanying bar graph. Linker genes were not included in the enrichment analysis. Pie chart representing significantly altered genes and pathways in PC.
Extended Data Figure 2 Selected genomic events in PC.
a, Lollipop plots showing the type and location of mutations in the RNA processing genes RBM10, SF3B1 and U2AF1 and the tumour suppressor TP53.In each plot, mutations observed across multiple cancers (top plot; PanCancer) are compared with those observed in the current study (bottom plot; PDAC). Significant recurrent mutations are labelled above the relevant lollipop. b, Regions of copy number alteration showing concordant gene expression changes. For each of the indicated chromosomes, significant GISTIC peaks are shown at their respective genomic locations (x axis) as grey bars. Each gene is represented by a dot at its specific chromosomal coordinate, with blue representing concordant copy number loss and gene downregulation and red representing concordant copy number amplification (copy number ≥ 8) and gene upregulation. Significance of concordant copy number/expression change is measured as a value of −log10 (q-value) times the sign of the direction of change. Dotted lines represent a significance threshold of −log10 (q-value = 0.05) times the sign of the direction of change. Genes showing concordant copy number/expression changes and overlapping GISTIC peaks are listed above the plot. Asterisk denotes known PC oncogenes showing amplification but non-significant concordant copy number/expression change.
Extended Data Figure 3 Classification of PC into 4 classes.
a, Unsupervised classification of PC RNAseq using NMF. Solutions are shown for k = 2 to k = 7 classes. A peak cophenetic correlation is observed for k = 4 classes. b, Silhouette information for k = 4 classes. c–e, Boxplots representing QPURE, stromal signature scores and immune signature scores stratified by class. Boxplots are annotated by a Kruskall–Wallis P value. For comparisons the following sample sizes were used: ADEX (n = 16); immunogenic (n = 25); squamous (n = 25); and pancreatic progenitor (n = 30). f, Heatmap showing differential gene expression between classes. Samples with positive silhouette widths were retained for ‘sam’ analysis. g, Heatmap showing overlap of the 4 classes identified in the current study and Collisson et al. classification27.
Extended Data Figure 4 Identification of 4 robust PC classes in 232 PCs with mixed low and high cellularity.
a, Unsupervised classification of PC expression array data representing 232 samples using NMF. Solutions are shown for k = 2 to k = 7 classes. b, Silhouette information for k = 4 classes. c, Heatmap showing differential gene expression between classes. d, Boxplots representing QPURE, stromal signature scores and immune signature scores stratified by class. e, Boxplots representing ADEX, pancreatic progenitor, squamous and immunogenic signature scores defined using the RNA-seq PC set stratified by class. Boxplots in d and e are annotated by a Kruskall–Wallis P value. For comparisons the following sample sizes were used: ADEX (n = 49); immunogenic (n = 67); squamous (n = 71); and pancreatic progenitor (n = 45).
Extended Data Figure 5 Characterization of PC subtypes.
a, Heatmap showing the statistical significance of correlations observed between the expressions of genes significantly expressed in each PC class and gene programmes identified by WGCNA. Pearson correlations and Student’s asymptotic P values are provided in each cell. b, Principal component analysis (PCA) using methylation data. Plot showing pairwise comparisons of samples distributed along the identified principle components (PC). Adjacent non-tumorous pancreatic samples represented as green points cluster as a distinct group. PC samples represented by points coloured brown (ADEX), blue (squamous), orange (pancreatic progenitor) and red (immunogenic) cluster together. c, Venn diagram showing the number of common and unique genes differentially methylated in the indicated PC subtypes when compared to adjacent non-tumorous pancreas. It is observed that distinct subsets of genes are differentially methylated in the 4 PC subtypes. d, Heatmap showing genes that are significantly methylated between tumours comprising the squamous class and all other classes. Methylation values for the same genes in adjacent non-tumorous pancreas are also shown. e–h, Plots showing regulation of gene expression by methylation. Hyper- or hypomethylation of the indicated probe is associated with either the concordant downregulation or upregulation of the indicated gene. Pearson correlation and adjusted P values are provided for each gene methylation comparison. Boxplot colours designate class: ADEX (brown); immunogenic (red); squamous (blue); and pancreatic progenitor (orange). Single letter designations representing the first letter of each class are provided under the relevant boxes in each plot.
Extended Data Figure 6 Core gene programmes (GP) defining the squamous class.
Each panel shows from left to right: (i) a heatmap representing the genes in the specified gene programme most correlated with the indicated PC class with tumours ranked according to their gene programme module eigengene values (MEs) (PC classes are designated by colour as follows: ADEX (brown); pancreatic progenitor (orange); immunogenic (red); and squamous (blue)); (ii) Kaplan–Meier analysis comparing survival of patients having either high or low gene programme MEs; (iii) pathways significantly enriched in a given GP functional interaction (FI) sub-network defined by the ReactomeFI Cytoscape plugin. P values represent FDR < 0.05.
Extended Data Figure 7 Gene programme defining the pancreatic progenitor class.
a, Panel showing from left to right: (i) a heatmap representing the genes in GP1 most correlated with the pancreatic progenitor class with tumours ranked according to their GP1 module eigengene values (MEs); (ii) Kaplan–Meier analysis comparing survival of patients having either high or low GP1 MEs; (iii) pathways significantly enriched in a GP1 FI sub-network defined by the ReactomeFI Cytoscape plugin. P values represent FDR <0.05. b, Network diagram depicting pathways significantly enriched in GP1 (FDR <0.0001). Different node colours indicate different network clusters or closely interconnected genes.
Extended Data Figure 8 Gene programmes defining the ADEX class.
a, b, Panel showing from left to right: (i) a heatmap representing the genes in the specified GP most correlated with the ADEX class with tumours ranked according to their GP module eigengene values (MEs); (ii) Kaplan–Meier analysis comparing survival of patients having either high or low GP MEs; (iii) pathways significantly enriched in a GP FI sub-network defined by the ReactomeFI Cytoscape plugin. P values represent FDR <0.05. c, Network diagram depicting pathways significantly enriched in GP9 (FDR <0.0001). Different node colours indicate different network clusters or closely interconnected genes. Genes comprising GP9 are indicated as coloured circles, whereas linker genes (genes not comprising GP9 but forming multiple connections in the network) are indicated as coloured diamonds. d, Network diagram depicting pathways significantly enriched in GP10 (FDR <0.0001). Different node colours indicate different network clusters or closely interconnected genes.
Extended Data Figure 9 Stratification of PC RNASeq data according to Moffitt et al.
a, Heatmap showing the stratification of the PC cohort of the current study using the tumour subtype classifier published in Moffitt et al.28. PCs were classified by consensus clustering using the top 50 weighted genes associated with the basal-like or classical subtypes. b, Boxplots showing the distribution of normal and activated stroma signature scores between the 4 PC classes identified in the current study. Boxplots are annotated by a Kruskall–Wallis P value. A significant difference in activated stroma signature scores was observed between squamous and ADEX tumours P value < 0.01 (t-test). Boxplot colours designate class: ADEX (brown); immunogenic (red); squamous (blue); and pancreatic progenitor (orange). c, Plots showing correlation between tumour cellularity, presented as a QPURE score, and either activated or normal stroma signature scores. Plots are annotated with Pearson correlation scores and significance values, with a linear fit represented by a solid line. Sample ICGC_0338, a rare acinar cell carcinoma is highlighted. This sample exhibits near 100% cellularity and has low activated or normal stroma signature scores. d, Principal component analysis (PCA) using methylation data. Plot showing pairwise comparisons of samples distributed along the identified principle components (PC). Adjacent non-tumorous pancreatic samples represented as green points cluster as a distinct group relative to ADEX samples (brown and red points). Rare acinar cell carcinomas (red) cluster with other ADEX samples (brown). All other PC samples are shown as grey points. e, Plot showing the correlation of expression of representative genes expressed in acinar cell carcinoma sample ICGC_0338 compared to the median expression of the same genes across all other ADEX samples. A red shaded region encompasses genes showing high median expression in all other ADEX but low expression in ICGC_0338. A brown shaded region encompasses genes showing high median expression in all other ADEX and correlatively high expression in ICGC_0338. Pearson’s correlation and significance are indicated.
Extended Data Figure 10 Gene programmes defining the immunogenic class.
a–c, Each panel shows from left to right: (i) a heatmap representing the genes in the specified gene programme most correlated with the indicated PC class with tumours ranked according to their gene programme module eigengene values (MEs). PC classes are designated by colour as follows: ADEX (brown); pancreatic progenitor (orange); immunogenic (red); and squamous (blue); (ii) Kaplan–Meier analysis comparing survival of patients having either high or low gene programme MEs; (iii) pathways significantly enriched in a given GP functional interaction (FI) sub-network defined by the ReactomeFI Cytoscape plugin. Corresponding Cytoscape files comprising GP ReactomeFI subnetworks are provided. d, Boxplot of immune gene expression stratified by class. Boxplots are annotated by a Kruskall–Wallis P value and box colours designate class: ADEX (brown); immunogenic (red); squamous (blue); and pancreatic progenitor (orange). Single letter designations representing the first letter of each class are provided under the relevant boxes in each plot.
Supplementary information
Supplementary Tables
This file contains Supplementary Tables 1-13 (XLSX 21586 kb)
Supplementary Tables
This file contains Supplementary Table 14 (XLSX 26694 kb)
Supplementary Tables
This file contains Supplementary Table 15 (XLSX 10181 kb)
Supplementary Tables
This file contains Supplementary Table 16 (XLSX 204 kb)
Supplementary Tables
This file contains Supplementary Table 17 (XLSX 31205 kb)
Supplementary Tables
This file contains Supplementary Table 18 (XLSX 63 kb)
Supplementary Tables
This file contains Supplementary Table 19 (XLSX 61164 kb)
Supplementary Tables
This file contains Supplementary Table 20 (XLSX 2555 kb)
Supplementary Tables
This file contains Supplementary Table 21 (XLSX 35 kb)
Supplementary Information
This file contains a list of the participants and their affiliations for the Australian Pancreatic Cancer Genome Initiative. (PDF 105 kb)
Rights and permissions
About this article
Cite this article
Bailey, P., Chang, D., Nones, K. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016). https://doi.org/10.1038/nature16965
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature16965
This article is cited by
-
Combination of mutations in genes controlling DNA repair and high mutational load plays a prognostic role in pancreatic ductal adenocarcinoma (PDAC): a retrospective real-life study in Sardinian population
Journal of Translational Medicine (2024)
-
K-Ras(V12) differentially affects the three Akt isoforms in lung and pancreatic carcinoma cells and upregulates E-cadherin and NCAM via Akt3
Cell Communication and Signaling (2024)
-
Pancreatic cancer acquires resistance to MAPK pathway inhibition by clonal expansion and adaptive DNA hypermethylation
Clinical Epigenetics (2024)
-
PAF1c links S-phase progression to immune evasion and MYC function in pancreatic carcinoma
Nature Communications (2024)
-
Therapeutic developments in pancreatic cancer
Nature Reviews Gastroenterology & Hepatology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.