Abstract
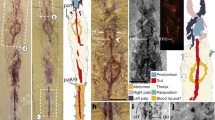
The molecularly defined clade Ecdysozoa1 comprises the panarthropods (Euarthropoda, Onychophora and Tardigrada) and the cycloneuralian worms (Nematoda, Nematomorpha, Priapulida, Loricifera and Kinorhyncha). These disparate phyla are united by their means of moulting, but otherwise share few morphological characters—none of which has a meaningful fossilization potential. As such, the early evolutionary history of the group as a whole is largely uncharted. Here we redescribe the 508-million-year-old stem-group onychophoran Hallucigenia sparsa2,3,4,5,6 from the mid-Cambrian Burgess Shale. We document an elongate head with a pair of simple eyes, a terminal buccal chamber containing a radial array of sclerotized elements, and a differentiated foregut that is lined with acicular teeth. The radial elements and pharyngeal teeth resemble the sclerotized circumoral elements and pharyngeal teeth expressed in tardigrades7,8,9, stem-group euarthropods10,11,12 and cycloneuralian worms13. Phylogenetic results indicate that equivalent structures characterized the ancestral panarthropod and, seemingly, the ancestral ecdysozoan, demonstrating the deep homology of panarthropod and cycloneuralian mouthparts, and providing an anatomical synapomorphy for the ecdysozoan supergroup.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aguinaldo, A. M. et al. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387, 489–493 (1997)
Conway Morris, S. A new metazoan from the Cambrian Burgess Shale of British Columbia. Palaeontology 20, 623–640 (1977)
Ramsköld, L. & Chen, J.-Y. in Arthropod Fossils and Phylogeny (ed. Edgecombe, G. D. ) 107–150 (Columbia Univ. Press, 1998)
Ramsköld, L. The second leg row of Hallucigenia discovered. Lethaia 25, 221–224 (1992)
Caron, J.-B., Smith, M. R. & Harvey, T. H. P. Beyond the Burgess Shale: Cambrian microfossils track the rise and fall of hallucigeniid lobopodians. Proc. R. Soc. Lond. B 280, 20131613 (2013)
Smith, M. R. & Ortega-Hernández, J. Hallucigenia’s onychophoran-like claws and the case for Tactopoda. Nature 514, 363–366 (2014)
Dewel, R. A. & Clark, W. H. Studies on the tardigrades. II. Fine structure of the pharynx of Milnesium tardigradum Doyère. Tissue Cell 5, 147–159 (1973)
Dewel, R. A. & Eibye-Jacobsen, J. The mouth cone and mouth ring of Echiniscus viridissimus Peterfi, 1956 (Heterotardigrada) with comparisons to corresponding structures in other tardigrades. Hydrobiologia 558, 41–51 (2006)
Guidetti, R. et al. Form and function of the feeding apparatus in Eutardigrada (Tardigrada). Zoomorphology 131, 127–148 (2012)
Vannier, J., Liu, J., Lerosey-Aubril, R., Vinther, J. & Daley, A. C. Sophisticated digestive systems in early arthropods. Nat. Commun. 5, 3641 (2014)
Budd, G. E. The morphology and phylogenetic significance of Kerygmachela kierkegaardi Budd (Buen Formation, Lower Cambrian, N Greenland). Trans. R. Soc. Edinb. Earth Sci. 89, 249–290 (1998)
Daley, A. C., Budd, G. E., Caron, J.-B., Edgecombe, G. D. & Collins, D. H. The Burgess Shale anomalocaridid Hurdia and its significance for early euarthropod evolution. Science 323, 1597–1600 (2009)
Conway Morris, S. Fossil priapulid worms. Spec. Pap. Pal. 20, (1977)
Budd, G. E. Tardigrades as ‘stem-group arthropods’: the evidence from the Cambrian fauna. Zool. Anz. 240, 265–279 (2001)
Eriksson, B. J. & Budd, G. E. Onychophoran cephalic nerves and their bearing on our understanding of head segmentation and stem-group evolution of Arthropoda. Arthropod Struct. Dev. 29, 197–209 (2000)
Elzinga, R. J. Microspines in the alimentary canal of Arthropoda, Onychophora, Annelida. Int. J. Ins. Morph. Emb. 27, 341–349 (1998)
Dewel, R. A. & Dewel, W. C. The brain of Echiniscus viridissimus Peterfi, 1956 (Heterotardigrada): A key to understanding the phylogenetic position of tardigrades and the evolution of the arthropod head. Zool. J. Linn. Soc. 116, 35–49 (1996)
Waggoner, B. The earliest evolutionary history of arthropods: what can the fossils tell us? Bol. Soc.. Entomológica Aragon. 26, 115–131 (1999)
Hou, X.-G. et al. Anomalocaris and other large animals in the lower Cambrian Chengjiang fauna of southwest China. GFF 117, 163–183 (1995)
Conway Morris, S. & Peel, J. S. New palaeoscolecidan worms from the Lower Cambrian: Sirius Passet, Latham Shale, and Kinzers Shale. Acta Pal. Pol. 55, 141–156 (2010)
Rota-Stabelli, O. et al. A congruent solution to arthropod phylogeny: phylogenomics, microRNAs and morphology support monophyletic Mandibulata. Proc. R. Soc. Lond. B 278, 298–306 (2011)
Martin, C. & Mayer, G. Neuronal tracing of oral nerves in a velvet worm—implications for the evolution of the ecdysozoan brain. Front. Neuroanat. 8, 7 (2014)
Dunn, C. W., Giribet, G., Edgecombe, G. D. & Hejnol, A. Animal phylogeny and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. 45, 371–395 (2014)
Liu, J. et al. New observations of the lobopod-like worm Facivermis from the Early Cambrian Chengjiang Lagerstätte. Chin. Sci. Bull. 51, 358–363 (2006)
Daley, A. C. & Bergström, J. The oral cone of Anomalocaris is not a classic ‘peytoia’. Naturwissenschaften 99, 501–504 (2012)
Maas, A., Huang, D., Chen, J.-Y., Waloszek, D. & Braun, A. Maotianshan-Shale nemathelminths—morphology, biology, and the phylogeny of Nemathelminthes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 254, 288–306 (2007)
Kristensen, R. M. Loricifera, a new phylum with Aschelminthes characters from the meiobenthos. Zeit. Zool. Syst. Evol. 21, 163–180 (1983)
Conway Morris, S. & Caron, J.-B. Pikaia gracilens Walcott, a stem-group chordate from the Middle Cambrian of British Columbia. Biol. Rev. Camb. Philos. Soc. 87, 480–512 (2012)
Smith, M. R. Nectocaridid ecology, diversity and affinity: early origin of a cephalopod-like body plan. Paleobiology 39, 297–321 (2013)
Goloboff, P. A., Farris, J. S. & Nixon, K. C. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786 (2008)
Goloboff, P. A. Estimating character weights during tree search. Cladistics 9, 83–91 (1993)
Walcott, C. D. Cambrian Geology and Paleontology II, no. 5. Middle Cambrian annelids. Smithson. Misc. Collect. 57, 109–144 (1911)
Ramsköld, L. & Hou, X.-G. New early Cambrian animal and onychophoran affinities of enigmatic metazoans. Nature 351, 225–228 (1991)
Hou, X.-G. & Bergström, J. Cambrian lobopodians–ancestors of extant onychophorans? Zool. J. Linn. Soc. 114, 3–19 (1995)
Steiner, M., Hu, S.-X., Liu, J. & Keupp, H. A new species of Hallucigenia from the Cambrian Stage 4 Wulongqing Formation of Yunnan (South China) and the structure of sclerites in lobopodians. Bull. Geosci. 87, 107–124 (2012)
Acknowledgements
We thank D. Erwin, M. Florence and P. Fenton for access to material and assistance with collections, G. Kretschmann and S. Whittaker for assistance with electron microscopy, and K. Meechan for assistance with data collection. ROM specimens were collected under Parks Canada Research and Collection permits to D. Collins. TNT is made available with the sponsorship of the Willi Hennig Society. Funding for this research was provided by Clare College, Cambridge (M.R.S.), NSERC Discovery Grant no. 341944 (J.-B.C.) and the ROM DMV Acquisition and Research Fund and Endowment Fund (J.-B.C.). This is ROM Burgess Shale project number 60.
Author information
Authors and Affiliations
Contributions
M.R.S. performed data analysis; both authors examined and imaged material and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
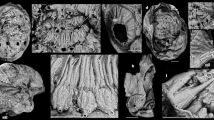
Extended Data Figure 1 Hallucigenia sparsa from the Burgess Shale.
a, b, largest (a, ROM 57169) and smallest (b, ROM 62093) specimens, to the same scale; c, ROM 57168, with enlargements of the anterior (d) and mid-trunk (e). Acronyms as in Fig. 1. Scale bars, 5 mm.
Extended Data Figure 2 Hallucigenia sparsa from the Burgess Shale.
a, ROM 63139, showing posterior body termination; b, c, NMNH 198658, showing posterior termination (see also Fig. 1e); d–g, ROM 63143: e, enlargement of region marked in d; f, g, backscatter SEMs of regions marked in e. Acronyms as in Fig. 1. Scale bars, 5 mm (a–d), 1 mm (e), 0.5 mm (f), 0.1 mm (g).
Extended Data Figure 3 Hallucigenia sparsa from the Burgess Shale.
a, c, ROM 63142: a, composite image incorporating part and counterpart of the entire specimen; c, claw pair. b, d–g, ROM 63051: b, composite image incorporating part and counterpart of the entire specimen; d, anterior section; e, f, eyes; g, claw pair. c–e are backscatter electron micrographs. Acronyms as in Fig. 1. Scale bars, 5 mm (a, b), 500 μm (d), 50 μm (c, f, g), 20 μm (e).
Extended Data Figure 4 Hallucigenia sparsa from the Burgess Shale.
a–d, ROM 61513: a, entire specimen; b–d, enlargements of anterior region, showing mouth opening, aciculae and eyes; mouth opening to right in b, to left in c, d. e, f, ROM 61143: anterior region marked in e is enlarged in f. Acronyms as in Fig. 1. Scale bars, 5 mm (a, e), 1 mm (b, f), 200 μm (c), 20 μm (d).
Extended Data Figure 5 Hallucigenia sparsa (ROM 62269) from the Burgess Shale.
a, part; b, counterpart, anterior section, showing eyes; c, d, eyes and mouthparts (backscatter SEM); e, f, detail of eyes (counterpart). Acronyms as in Fig. 1. Scale bars, 1 mm (a), 400 μm (b), 200 μm (e), 100 μm (c, d), 20 μm (f).
Extended Data Figure 6 Hallucigenia sparsa from the Burgess Shale.
a–d, NMNH 83935 (holotype): in contrast to body tissue, decay fluids lack a sharp margin and are non-reflective; e, f, ROM 57776, showing full length of appendage one. Acronyms as in Fig. 1. Scale bars, 5 mm.
Extended Data Figure 7 Hallucigenia sparsa (ROM 63146), composite image of part and counterpart.
Acronyms as in Fig. 1. Scale bar, 5 mm.
Extended Data Figure 8 Hallucigenia sparsa from the Burgess Shale.
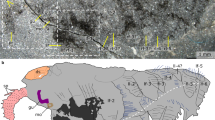
a–d, NMNH 193996: b, c, enlargements of area boxed in a; c, secondary electron micrograph; d, backscatter electron micrograph of region marked in c. e–g, ROM 63141, showing position of mouth. h–j, ROM 63144: i, secondary electron image of region marked in h; j, backscatter electron image of region marked in i, showing eyes and mouthparts, with interpretative diagram. k–m, ROM 63140: l, backscatter SEM of head, showing right eye and mouthparts (enlarged in m, with interpretative diagram). Acronyms as in Fig. 1. Scale bars, 10 mm (k), 5 mm (a, e, h), 1 mm (b, c, l), 0.5 mm (i), 0.1 mm (d, j, m).
Extended Data Figure 9 Hallucigenia sparsa from the Burgess Shale.
a, ROM 43045, cluster of dissociated specimens; b, ROM 63145, dissociated specimen showing spines in close anatomical position. Acronyms as in Fig. 1. Scale bars, 10 mm.
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-2, which detail the character coding for phylogenetic analysis, and transformations implied by our most parsimonious tree. (PDF 691 kb)
Supplementary Table 1
This table details examined specimens of Hallucigenia sparsa. (XLS 49 kb)
Supplementary Table 2
This table details and tabulates measurements recorded from Hallucigenia specimens. (XLSX 181 kb)
Supplementary Data
This zipped file contains the Phylogeny data files comprising character matrix in Nexus and Excel formats, most parsimonious trees for all values of k and TNT script used to generate trees. (ZIP 188 kb)
Three dimensional turntable reconstruction of Hallucigenia sparsa.
Rotating three-dimensional model of Hallucigenia sparsa, depicting anatomical interpretations. By Lars Fields. (MOV 24882 kb)
Three dimensional life reconstruction of Hallucigenia sparsa.
Reconstruction of Hallucigenia soft anatomy, showing a possible interpretation of walking style and trunk flexibility. Reconstruction by Lars Fields. (MOV 33554 kb)
Rights and permissions
About this article
Cite this article
Smith, M., Caron, JB. Hallucigenia’s head and the pharyngeal armature of early ecdysozoans. Nature 523, 75–78 (2015). https://doi.org/10.1038/nature14573
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14573
This article is cited by
-
Ancestral morphology of Ecdysozoa constrained by an early Cambrian stem group ecdysozoan
BMC Evolutionary Biology (2020)
-
Brain and eyes of Kerygmachela reveal protocerebral ancestry of the panarthropod head
Nature Communications (2018)
-
A Cambrian unarmoured lobopodian, †Lenisambulatrix humboldti gen. et sp. nov., compared with new material of †Diania cactiformis
Scientific Reports (2018)
-
The functional head of the Cambrian radiodontan (stem-group Euarthropoda) Amplectobelua symbrachiata
BMC Evolutionary Biology (2017)
-
Cambrian suspension-feeding lobopodians and the early radiation of panarthropods
BMC Evolutionary Biology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.