Abstract
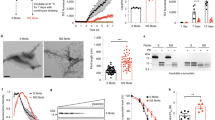
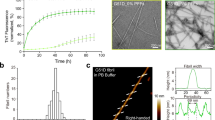
Misfolded protein aggregates represent a continuum with overlapping features in neurodegenerative diseases, but differences in protein components and affected brain regions1. The molecular hallmark of synucleinopathies such as Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy are megadalton α-synuclein-rich deposits suggestive of one molecular event causing distinct disease phenotypes. Glial α-synuclein (α-SYN) filamentous deposits are prominent in multiple system atrophy and neuronal α-SYN inclusions are found in Parkinson’s disease and dementia with Lewy bodies2. The discovery of α-SYN assemblies with different structural characteristics or ‘strains’ has led to the hypothesis that strains could account for the different clinico-pathological traits within synucleinopathies3,4. In this study we show that α-SYN strain conformation and seeding propensity lead to distinct histopathological and behavioural phenotypes. We assess the properties of structurally well-defined α-SYN assemblies (oligomers, ribbons and fibrils) after injection in rat brain. We prove that α-SYN strains amplify in vivo. Fibrils seem to be the major toxic strain, resulting in progressive motor impairment and cell death, whereas ribbons cause a distinct histopathological phenotype displaying Parkinson’s disease and multiple system atrophy traits. Additionally, we show that α-SYN assemblies cross the blood–brain barrier and distribute to the central nervous system after intravenous injection. Our results demonstrate that distinct α-SYN strains display differential seeding capacities, inducing strain-specific pathology and neurotoxic phenotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lee, S. J., Desplats, P., Sigurdson, C., Tsigelny, I. & Masliah, E. Cell-to-cell transmission of non-prion protein aggregates. Nature Rev. Neurol. 6, 702–706 (2010)
Goedert, M., Clavaguera, F. & Tolnay, M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 33, 317–325 (2010)
Guo, J. L. et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154, 103–117 (2013)
Bousset, L. et al. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 4, 2575 (2013)
Li, J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature Med. 14, 501–503 (2008)
Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B. & Olanow, C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nature Med. 14, 504–506 (2008)
Lee, H. J., Bae, E. J. & Lee, S. J. Extracellular α-synuclein—a novel and crucial factor in Lewy body diseases. Nature Rev. Neurol. 10, 92–98 (2014)
Conway, K. A. et al. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc. Natl Acad. Sci. USA 97, 571–576 (2000)
Winner, B. et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc. Natl Acad. Sci. USA 108, 4194–4199 (2011)
Pieri, L., Madiona, K., Bousset, L. & Melki, R. Fibrillar alpha-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys. J. 102, 2894–2905 (2012)
Luk, K. C. et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012)
Sacino, A. N. et al. Induction of CNS alpha-synuclein pathology by fibrillar and non-amyloidogenic recombinant alpha-synuclein. Acta Neuropathol. Commun. 1, 38 (2013)
Ulusoy, A., Decressac, M., Kirik, D. & Bjorklund, A. Viral vector-mediated overexpression of alpha-synuclein as a progressive model of Parkinson's disease. Prog. Brain Res. 184, 89–111 (2010)
Van der Perren, A. et al. Longitudinal follow-up and characterization of a robust rat model for Parkinson's disease based on overexpression of alpha-synuclein with adeno-associated viral vectors. Neurobiol. Aging 36, 1543–1558 (2015)
Rey, N. L., Petit, G. H., Bousset, L., Melki, R. & Brundin, P. Transfer of human alpha-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol. 126, 555–573 (2013)
Reyes, J. F. et al. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia 62, 387–398 (2014)
Janezic, S. et al. Deficits in dopaminergic transmission precede neuron loss and dysfunction in a new Parkinson model. Proc. Natl Acad. Sci. USA 110, E4016–E4025 (2013)
Milber, J. M. et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology 79, 2307–2314 (2012)
Kramer, M. L. & Schulz-Schaeffer, W. J. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J. Neurosci. 27, 1405–1410 (2007)
Wilhelm, B. G. et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344, 1023–1028 (2014)
Greten-Harrison, B. et al. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl Acad. Sci. USA 107, 19573–19578 (2010)
Buell, A. K. et al. Solution conditions determine the relative importance of nucleation and growth processes in alpha-synuclein aggregation. Proc. Natl Acad. Sci. USA 111, 7671–7676 (2014)
Brundin, P., Melki, R. & Kopito, R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nature Rev. Mol. Cell Biol. 11, 301–307 (2010)
Mabbott, N. A. & MacPherson, G. G. Prions and their lethal journey to the brain. Nature Rev. Microbiol. 4, 201–211 (2006)
Sacino, A. N. et al. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl Acad. Sci. USA 111, 10732–10737 (2014)
Holmqvist, S. et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128, 805–820 (2014)
Redeker, V., Pemberton, S., Bienvenut, W., Bousset, L. & Melki, R. Identification of protein interfaces between alpha-synuclein, the principal component of Lewy bodies in Parkinson disease, and the molecular chaperones human Hsc70 and the yeast Ssa1p. J. Biol. Chem. 287, 32630–32639 (2012)
Van der Perren, A. et al. Efficient and stable transduction of dopaminergic neurons in rat substantia nigra by rAAV 2/1, 2/2, 2/5, 2/6.2, 2/7, 2/8 and 2/9. Gene Ther. 18, 517–527 (2011)
Kahle, P. J. et al. Selective insolubility of alpha-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am. J. Pathol. 159, 2215–2225 (2001)
Luk, K. C. et al. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J. Exp. Med. 209, 975–986 (2012)
Oliveras-Salvá, M. et al. rAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol. Neurodegener. 8, 44 (2013)
Baekelandt, V. et al. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum. Gene Ther. 13, 841–853 (2002)
Schmitz, C. & Hof, P. R. Design-based stereology in neuroscience. Neuroscience 130, 813–831 (2005)
Reinartz, S., Biro, I., Gal, A., Giugliano, M. & Marom, S. Synaptic dynamics contribute to long-term single neuron response fluctuations. Front. Neural Circuits 8, 71 (2014)
Linaro, D., Couto, J. & Giugliano, M. Command-line cellular electrophysiology for conventional and real-time closed-loop experiments. J. Neurosci. Methods 230, 5–19 (2014)
Mahmud, M., Pulizzi, R., Vasilaki, E. & Giugliano, M. QSpike tools: a generic framework for parallel batch preprocessing of extracellular neuronal signals recorded by substrate microelectrode arrays. Front. Neuroinform. 8, 26 (2014)
Acknowledgements
We thank J. Van Asselbergs and C. van Heijningen for their technical assistance and Z. Debyser for revising the manuscript. We thank J. Hofkens and C. David for the use of the CLSM and V. Redeker for assessing the number of atto dyes bound to α-SYN by mass spectrometry. The authors thank the Leuven Viral Vector Core (LVVC) for the rAAV vector construction, optimization and production. M.G., R.P., and A.M. thank M. Wijnants and D. Van Dyck for their excellent technical assistance. Research was funded by the FWO-Vlaanderen (G.0768.10, G.0927.14 and PhD fellowship to W.P.), the IWT-Vlaanderen (IWT SBO/80020 Neuro-TARGET, SBO/110068 OPTOBRAIN and SBO/130065 MIRIAD), the FP7 RTD projects MEFOPA (HEALTH-2009-241791) and INMiND (HEALTH-F2-2011-278850), the KU Leuven (OT/08/052A, OT/14/120, IMIR PF/10/017), the Agence Nationale de la Recherche (ANR-09-MNPS-013-01 and ANR-11-BSV8-021-01), the Centre National de la Recherche Scientifique, a ‘Coup d’Elan à la Recherche Française’ award from Fondation Bettencourt Schueller, the EC-FP7 (Marie Curie Initial Training Network “NAMASEN”, grant n. 264872, the ICT-FET projects “ENLIGHTENMENT” and “BRAINLEAP”, grants no. 284801 and 306502), and the Belgian Science Policy Office (grant no. IUAP-VII/20). The authors declare that there is no actual or potential conflict of interest.
Author information
Authors and Affiliations
Contributions
W.P. designed and conducted in vivo experiments. L.B. generated α-SYN assemblies and performed characterization of α-SYN strains. A.V.P. conducted in vivo experiments. C.V.H. was responsible for viral vector production. R.M. co-designed the study. V.B. was responsible for the overall design and coordination of the study. M.G., R.P., and A.M conducted intracellular and extracellular in vitro experiments and analysed the electrophysiological recordings. W.P., V.B. and R.M. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Characterization of α-SYN assemblies.
a–d, Electron micrographs of α-SYN monomers (a), oligomers (b), fibrils (c) and ribbons (d) used throughout this study. The scale bars represent 200 nm. e, Quantification of endotoxin amounts in different recombinant α-SYN preparations used throughout this study. Endotoxin levels were below 0.05 units per injection for all conditions. f–h, Mass spectrometry analysis of covalently labelled fluorescent wild-type (WT) α-SYN by MALDI-TOF. f, Unlabelled WT α-SYN (theoretical mass 14,460.1 Da); g, atto-550 WT α-SYN (average molecular ratio 1 atto molecule per α-SYN molecule) and h, atto-647 WT α-SYN (average molecular ratio 1 atto molecule per α-SYN molecule).
Extended Data Figure 2 Trans-synaptic spreading of fluorescently labelled α-SYN in rat striatum.
α-SYN assemblies were injected in rat substantia nigra (red injection spot) and were allowed to spread for 7 days to the striatum. Sagittal and coronal views are depicted in a and b, respectively. Representative confocal images of α-SYN spreading in c, dopaminergic neurons and d, striatal dopaminergic axons for α-SYN oligomers, ribbons and fibrils after 7 days. Reconstructed images from z-stack tiles of the dopaminergic neurons and axons in striatum were rendered into complete axonal reconstructions by tiling the entire set of z-stacks. Scale bar is 20 μm. The rectangle identifying Atto-550-tagged α-SYN is shown at a higher magnification in upper right corner of sections in lower panels. e, Representative confocal image of recombinant oligomeric Atto-550 labelled α-SYN uptake in medium spiny neurons (MSN) after injection in substantia nigra. White arrows indicate positive inclusions. f, Quantification of fluorescently labelled α-SYN DARPP-32+ MSN. Uptake is measured after 1 and 7 days for oligomers (n1 day = 1,674, n7 days = 2,118, nanimals = 3), ribbons (n1 day = 2,152, n7 days = 1,968, nanimals = 3) and fibrils (n1 day = 1,223, n7 days = 1,486, nanimals = 3). Oligomers show increased uptake rates compared to ribbons and fibrils (P < 0.001, P < 0.01, one-way ANOVA with Dunnet’s multiple comparison test for 7 day time point) and increases over time (###P < 0.001, one-way ANOVA with Tukey’s multiple comparison test). Error bars indicate s.e.m., scale bars indicate 20 μm. g, Correlation of spreading volume and trans-synaptic spread at 7 days. Oligomers show the highest spreading capacity compared to fibrils and ribbons using two independent techniques and experiments.
Extended Data Figure 3 Histopathological hallmarks after nigral inoculation of α-SYN strains.
a–c, Immunofluorescent staining in substantia nigra shows a, granular cytoplasmic, b, intracytoplasmic and c, nuclear Pα-SYN inclusions in dopaminergic neurons. Reconstructed images from z-stack tiles were rendered into complete reconstructions by tiling the entire set of z-stacks. Scale bars indicate 20 μm.
Extended Data Figure 4 Phosphorylation pattern of different α-SYN assemblies upon rAAV-driven α-SYN overexpression.
a, Inoculation of ribbons and fibrils result in increased amount of phosphorylated α-SYN cells in substantia nigra. Scale bar, 50 μm. b, Animals where α-SYN overexpression is rAAV-driven yield a total of 12,426 ± 1,288 cells with phosphorylated α-SYN (s.e.m, n = 4). A significant increase of cells with phosphorylated α-SYN is observed upon injection of ribbons and fibrils, to 22,254 cells ± 2,800 (s.e.m, n = 4) and 19,690 cells ± 1,803 (s.e.m, n = 4), respectively (P < 0.01, P < 0.05, one-way ANOVA with Dunnet’s multiple comparison test versus control, #P < 0.05, one-way ANOVA with Tukey’s multiple comparison test).
Extended Data Figure 5 Intracellular and extracellular electrophysiological recordings of early effects of α-SYN assemblies on the electrical properties of single neurons.
Rat primary cortical neurons, cultured ex vivo for over 4 weeks, displayed no significant alterations of the intrinsic electrical phenotype after exposure for 3–4 days to 1 µM of α-SYN assemblies. This was characterized by means of intracellular recordings, and analysed in current-clamp in terms of membrane passive properties (n = 32 cells), a, as membrane time constant, b, membrane apparent input resistance, c, resting membrane potential and e, as evoked excitable responses (n = 30 cells). By the same technique, the amplitude of d, excitatory spontaneous post-synaptic currents (PSCs) revealed a significant downregulation, recorded under voltage-clamp (n = 32 cells).
Extended Data Figure 6 α-SYN strains propagate after intracerebral inoculation.
a, Preformed α-SYN fibrils and ribbons resist 1% sarkosyl treatment and are pelleted upon sedimentation at 200,000g for 60 min at 25 °C in vitro. (T, total; S, Soluble; P, Pellet) b, Fibrils resist 1% sarkosyl treatment and persist more after striatal injections than ribbons or oligomers. Sarkosyl-insoluble α-SYN from animals inoculated fibrils or ribbons is phosphorylated. Mean and associated standard error was calculated from 3 independent quantifications of the intensities made using ImageJ on samples from two independent experiments.
Extended Data Figure 7 α-SYN strains recruit rodent α-SYN.
Seeding of endogenous α-SYN in rat brain by exogenous human ribbons, fibrils or diseased rat brain homogenates. PBS was inoculated to the control animal. The nature of α-SYN assemblies was assessed by western blot following native PAGE separation prior (−) or after (+) proteinase K (76 ng µl−1) treatment. a, Staining with rat-specific antibody (D37A6) detects endogenous α-SYN and b, staining with human-specific antibody (Syn211) detects exogenous injected α-SYN on a duplicate blot. Arrows indicate fibrillar α-SYN at the bottom of the gel well. The non specific band (*) is recognized by the secondary antibody. Representative image from 3 independent experiments is shown.
Extended Data Figure 8 α-SYN fibrils persist months after intracerebral inoculation.
a, α-SYN assemblies were injected in rat substantia nigra (red injection spot) and assessed 4 months later in striatum. b, Immunohistochemical analysis of rat striatum reveals clear human α-SYN+ inclusions colocalizing with dopaminergic axons. Representative image of fibrils is shown with detailed image in right upper corner. Right panel shows pseudocolour image of only human α-SYN. Scale bar indicates 30 μm. c, Quantification of different α-SYN strains in rat striatum by means of total fluorescent units. Fibrils appear to persist to a much higher extent compared to ribbons (###P < 0.001, one-way ANOVA with Tukey’s multiple comparison test, n = 4). Oligomers and brain homogenate show very low immunofluorescent staining for human-specific α-SYN. d, Fibrils appear as axonal inclusions and are abundantly present in striatum in contrast to ribbons after nigral injection. Oligomers and brain homogenate are not detected using the same human-specific antibody Syn211. Scale bar indicates 40 μm.
Extended Data Figure 9 α-SYN ribbons and fibrils spread across the central nervous system after intravenous administration.
Overview of human α-SYN (Syn211) inclusions in different areas of the central nervous system after intravenous injection of α-SYN oligomers, ribbons and fibrils. Scale bars indicate 50 μm.
Extended Data Figure 10 Intravenous injection of α-SYN strains leads to increased microglial response and distinct histopathological hallmarks in the spinal cord.
Quantification of human α-SYN (huα-SYN) density and Mac1/CD11b microglial response following intravenous administration of a saline solution or α-SYN oligomers, ribbons or fibrils in a, b, cervical, c, d, thoracic and e, f, lumbosacral segments (P < 0.05, one-way ANOVA with Dunnet’s multiple comparison test versus control, ###P < 0.001, ##P < 0.01, #P < 0.05, one-way ANOVA with Tukey’s multiple comparison test, n = 4). Error bars indicate s.e.m., white boxes and grey boxes indicate 60 and 120 days after intravenous injections, respectively. g, Immunohistochemical staining for growth associated protein 43 (GAP43), an early injury marker, shows a filamentous distribution for α-SYN ribbons and cellular distribution for α-SYN fibrils (white arrow heads). Scale bars indicate 50 μm.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3. (PDF 233 kb)
Rights and permissions
About this article
Cite this article
Peelaerts, W., Bousset, L., Van der Perren, A. et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 (2015). https://doi.org/10.1038/nature14547
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14547
This article is cited by
-
Parkinson’s disease-derived α-synuclein assemblies combined with chronic-type inflammatory cues promote a neurotoxic microglial phenotype
Journal of Neuroinflammation (2024)
-
Neuropathology of incidental Lewy body & prodromal Parkinson’s disease
Molecular Neurodegeneration (2023)
-
The G51D SNCA mutation generates a slowly progressive α-synuclein strain in early-onset Parkinson’s disease
Acta Neuropathologica Communications (2023)
-
AAV-mediated expression of a new conformational anti-aggregated α-synuclein antibody prolongs survival in a genetic model of α-synucleinopathies
npj Parkinson's Disease (2023)
-
Intrastriatal injection of Parkinson’s disease intestine and vagus lysates initiates α-synucleinopathy in rat brain
Cell Death & Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.