Abstract
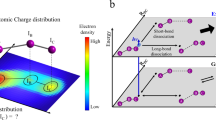
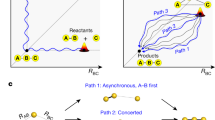
The making and breaking of atomic bonds are essential processes in chemical reactions. Although the ultrafast dynamics of bond breaking have been studied intensively using time-resolved techniques1,2,3, it is very difficult to study the structural dynamics of bond making, mainly because of its bimolecular nature. It is especially difficult to initiate and follow diffusion-limited bond formation in solution with ultrahigh time resolution. Here we use femtosecond time-resolved X-ray solution scattering to visualize the formation of a gold trimer complex, in real time without the limitation imposed by slow diffusion. This photoexcited gold trimer, which has weakly bound gold atoms in the ground state4,5,6, undergoes a sequence of structural changes, and our experiments probe the dynamics of individual reaction steps, including covalent bond formation, the bent-to-linear transition, bond contraction and tetramer formation with a time resolution of ∼500 femtoseconds. We also determined the three-dimensional structures of reaction intermediates with sub-ångström spatial resolution. This work demonstrates that it is possible to track in detail and in real time the structural changes that occur during a chemical reaction in solution using X-ray free-electron lasers7 and advanced analysis of time-resolved solution scattering data.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zewail, A. H. Laser femtochemistry. Science 242, 1645–1653 (1988)
Harris, A. L., Brown, J. K. & Harris, C. B. The nature of simple photodissociation reactions in liquids on ultrafast time scales. Annu. Rev. Phys. Chem. 39, 341–366 (1988)
Jonas, D. M., Bradforth, S. E., Passino, S. A. & Fleming, G. R. Femtosecond wavepacket spectroscopy: influence of temperature, wavelength, and pulse duration. J. Phys. Chem. 99, 2594–2608 (1995)
Rawashdeh-Omary, M. A., Omary, M. A., Patterson, H. H. & Fackler, J. P. Excited-state interactions for [Au(CN)2−]n and [Ag(CN)2−]n oligomers in solution. Formation of luminescent gold-gold bonded excimers and exciplexes. J. Am. Chem. Soc. 123, 11237–11247 (2001)
Iwamura, M., Nozaki, K., Takeuchi, S. & Tahara, T. Real-time observation of tight Au–Au bond formation and relevant coherent motion upon photoexcitation of [Au(CN)2−] oligomers. J. Am. Chem. Soc. 135, 538–541 (2013)
Cui, G. L., Cao, X. Y., Fang, W. H., Dolg, M. & Thiel, W. Photoinduced gold(I)-gold(I) chemical bonding in dicyanoaurate oligomers. Angew. Chem. Int. Ed. 52, 10281–10285 (2013)
Tamasaku, K. et al. X-ray two-photon absorption competing against single and sequential multiphoton processes. Nature Photon. 8, 313–316 (2014)
Rini, M., Magnes, B. Z., Pines, E. & Nibbering, E. T. J. Real-time observation of bimodal proton transfer in acid-base pairs in water. Science 301, 349–352 (2003)
Rosspeintner, A., Lang, B. & Vauthey, E. Ultrafast photochemistry in liquids. Annu. Rev. Phys. Chem. 64, 247–271 (2013)
Pyykkö, P. Theoretical chemistry of gold. Angew. Chem. Int. Ed. 43, 4412–4456 (2004)
Wang, S. G. & Schwarz, W. H. E. Quasi-relativistic density functional study of aurophilic interactions. J. Am. Chem. Soc. 126, 1266–1276 (2004)
Schmidbaur, H. & Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 37, 1931–1951 (2008)
Ihee, H. Visualizing solution-phase reaction dynamics with time-resolved X-ray liquidography. Acc. Chem. Res. 42, 356–366 (2009)
Ihee, H. et al. Ultrafast X-ray diffraction of transient molecular structures in solution. Science 309, 1223–1227 (2005)
Davidsson, J. et al. Structural determination of a transient isomer of CH2I2 by picosecond x-ray diffraction. Phys. Rev. Lett. 94, 245503 (2005)
Christensen, M. et al. Time-resolved X-ray scattering of an electronically excited state in solution. Structure of the 3A2u state of tetrakis-μ-pyrophosphitodiplatinate(II). J. Am. Chem. Soc. 131, 502–508 (2009)
Kim, K. H. et al. Solvent-dependent molecular structure of ionic species directly measured by ultrafast X-ray solution scattering. Phys. Rev. Lett. 110, 165505 (2013)
Plech, A., Kotaidis, V., Lorenc, M. & Boneberg, J. Femtosecond laser near-field ablation from gold nanoparticles. Nature Phys. 2, 44–47 (2006)
Kim, K. H. et al. Direct observation of cooperative protein structural dynamics of homodimeric hemoglobin from 100 ps to 10 ms with pump-probe X-ray solution scattering. J. Am. Chem. Soc. 134, 7001–7008 (2012)
Sokolowski-Tinten, K. et al. Femtosecond X-ray measurement of coherent lattice vibrations near the Lindemann stability limit. Nature 422, 287–289 (2003)
Fritz, D. M. et al. Ultrafast bond softening in bismuth: mapping a solid’s interatomic potential with X-rays. Science 315, 633–636 (2007)
Coppens, P. Molecular excited-state structure by time-resolved pump-probe X-ray diffraction. What is new and what are the prospects for further progress? J. Phys. Chem. Lett. 2, 616–621 (2011)
Miller, T. A. et al. The mechanism of ultrafast structural switching in superionic copper(I) sulphide nanocrystals. Nature Commun. 4, 1369 (2013)
Zewail, A. H. Four-dimensional electron microscopy. Science 328, 187–193 (2010)
Kirchner, F. O., Gliserin, A., Krausz, F. & Baum, P. Laser streaking of free electrons at 25 keV. Nature Photon. 8, 52–57 (2014)
Miller, R. J. D. Mapping atomic motions with ultrabright electrons: the chemists’ gedanken experiment enters the lab frame. Annu. Rev. Phys. Chem. 65, 583–604 (2014)
Bressler, C. et al. Femtosecond XANES study of the light-induced spin crossover dynamics in an iron(II) complex. Science 323, 489–492 (2009)
Lemke, H. T. et al. Femtosecond X-ray absorption spectroscopy at a hard X-ray free electron laser: application to spin crossover dynamics. J. Phys. Chem. A 117, 735–740 (2013)
Zhang, W. et al. Tracking excited-state charge and spin dynamics in iron coordination complexes. Nature 509, 345–348 (2014)
Arnlund, D. et al. Visualizing a protein quake with time-resolved X-ray scattering at a free-electron laser. Nature Methods 11, 923–926 (2014)
Inubushi, Y. et al. Determination of the pulse duration of an X-ray free electron laser using highly resolved single-shot spectra. Phys. Rev. Lett. 109, 144801 (2012)
Ishikawa, T. et al. A compact X-ray free-electron laser emitting in the sub-angstrom region. Nature Photon. 6, 540–544 (2012)
Rawashdeh-Omary, M. A., Omary, M. A. & Patterson, H. H. Oligomerization of Au(CN)2− and Ag(CN)2− ions in solution via ground-state aurophilic and argentophilic bonding. J. Am. Chem. Soc. 122, 10371–10380 (2000)
Kim, T. K., Lee, J. H., Wulff, M., Kong, Q. Y. & Ihee, H. Spatiotemporal kinetics in solution studied by time-resolved X-ray liquidography (solution scattering). ChemPhysChem 10, 1958–1980 (2009)
Ichiyanagi, K. et al. 100 ps time-resolved solution scattering utilizing a wide-bandwidth X-ray beam from multilayer optics. J. Synchrotron Radiat. 16, 391–394 (2009)
Haldrup, K. et al. Structural tracking of a bimolecular reaction in solution by time-resolved X-ray scattering. Angew. Chem. Int. Ed. 48, 4180–4184 (2009)
Jun, S. et al. Photochemistry of HgBr2 in methanol investigated using time-resolved X-ray liquidography. Phys. Chem. Chem. Phys. 12, 11536–11547 (2010)
Acknowledgements
We thank M. Iwamura and K. Nozaki for discussions. This work was supported by IBS-R004-G2; the X-ray Free Electron Laser Priority Strategic Program of MEXT, Japan; PRESTO/JST; the Innovative Areas ‘Artificial Photosynthesis (AnApple)’ (no. 25107527) grant from the Japan Society for the Promotion of Science; and the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1002511). The experiments were performed at beamline BL3 of SACLA with the approval of the Japan Synchrotron Radiation Research Institute (proposal nos 2012A8030, 2012A8038, 2012B8029, 2012B8043, 2013A8053, 2013B8036, 2013B8059, 2014A8042 and 2014A8022) and at beamline NW14A of KEK with the approval of the Photon Factory Program Advisory Committee (proposal nos 2011G655, 2012G778 and 2012G779).
Author information
Authors and Affiliations
Contributions
H.I. and S.-i.A. designed the study. K.H.K., J.G.K., S.N., Tokushi Sato, K.Y.O., T.W.K., H.K., J.J., S.P., C.S., Takahiro Sato, K.O., T.T., K.T., M.Y., T.I., Jeongho Kim, H.I. and S.-i.A. did the experiment. K.H.K., J.G.K., S.N., Tokushi Sato and Joonghan Kim analysed the data. K.H.K., J.G.K., S.N., K.Y.O., R.R., Jeongho Kim, H.I. and S.-i.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Comparison of the TRXSS signals at SACLA and KEK and the TRXSS data in the entire time range.
a, Comparison of the difference scattering curves at 100 ps time delay measured at SACLA (black) and KEK (red). The error bar at each data point indicates the standard error determined from 50 independent measurements. The two curves are nearly identical to each other within the experimental error, indicating that the difference scattering curves are highly reproducible and independent of the facility. b, Experimental difference scattering curves, qΔS(q, t), in the entire time range from –800 fs to 1 μs.
Extended Data Figure 2 Solvent heating contribution to the TRXSS signal.
a, Experimental difference scattering curves, qΔS(q), of FeCl3 solution measured at several time delays (400 fs, 1.9 ps, 3.9 ps, 5.9 ps, 7.9 ps, 30 ps and 100 ps). b, SVD of the experimental difference scattering curves of FeCl3 measured from –10 ps to 100 ps. The first two right singular vectors multiplied by singular values are shown. c, The first right singular vector (black circles) fitted by an error function (red curve). This result implies that only a single difference scattering curve accounts for solvent heating in the time range up to 100 ps. d, Comparison of the difference scattering curve of the [Au(CN)2–]3 solution at 1 μs time delay (black) and the difference scattering curve for solvent heating (red). The error bar at each data point indicates the standard error determined from 50 independent measurements. At this time delay, the two curves are almost identical to each other within the experimental error, confirming that the difference scattering at late time delays are dominated by the solvent heating.
Extended Data Figure 3 Difference RDFs in real space.
Difference RDFs, r2ΔS(r), obtained by Fourier sine transformation of qΔS(q).
Extended Data Figure 4 Species-associated difference RDFs of the transient states.
The species-associated difference RDFs of the S0, S1, T1 and tetramer states correspond to  ,
,  ,
,  and
and  , respectively. We used a common S0 structure when fitting all four species-associated difference RDFs. By optimizing the fit between the theoretical and the experimental difference RDFs for each transient species via the structural fitting analysis, we were able to obtain the theoretical RDF of the S0 state.
, respectively. We used a common S0 structure when fitting all four species-associated difference RDFs. By optimizing the fit between the theoretical and the experimental difference RDFs for each transient species via the structural fitting analysis, we were able to obtain the theoretical RDF of the S0 state.
Extended Data Figure 5 Radial distribution functions, r2S(r, t).
The RDF of the S0 state was added to the RDFs at all time delays to emphasize only the contributions of the transient solute species associated with the bond formation.
Extended Data Figure 6 Comparison of the scattering from Au atoms and other contributions.
a, Because the scattering intensities from C and N atoms are negligibly small, the total scattering pattern is almost the same as the scattering from Au atoms only. b, The contribution of the cage term is small and the total scattering pattern is therefore almost the same as the solute-only term.
Extended Data Figure 7 Contributions of trimer and dimer to X-ray scattering signal.
a, Concentrations of the three species [Au], [Au2] and [Au3], calculated as a function of c, which is the initial concentration of monomers of the gold complex. We assumed that K2 is 10 M–2 in this case. b, Absorption spectra of aqueous solutions of K[Au(CN)2] at various concentrations measured with a 0.5 mm path length cell. Four points (A1, A2, A3 and A4) that are used as inputs are indicated. c, Theoretical difference scattering curves for the trimer (black) and the dimer (red). Relative intensities of the two curves were estimated realistically based on the excitation probabilities and the equilibrium of the two species.
Extended Data Figure 8 Comparison of the TRXSS data measured with excitations at two different wavelengths, 310 and 267 nm.
a, Right singular vectors obtained from the SVD analysis for the 310 nm (left) and 267 nm (right) excitations. For both cases, the right singular vectors were fitted by only one kinetic component and their extracted time constants are almost identical to each other. b, Difference scattering curves at 100 ps time delay measured with excitations at 310 nm (black) and 267 nm (red). The error bar at each data point indicates the standard error determined from 50 independent measurements. The two curves are identical to each other within the experimental error. This similarity between the kinetics and the shapes of the difference scattering curves indicates negligible contribution from dimer excitation for both 310 and 267 nm excitations.
Extended Data Figure 9 Mechanism of photoinduced bond formation in [Au(CN)2–]3. Results from our TRXSS data (red) and the previous transient absorption experiment (blue) are shown together.
Our findings are in good agreement with the reaction mechanism proposed in the transient absorption study, except for the structural assignments of the early kinetics. Considering that TRXSS is sensitive only to the processes accompanying structural change, the intersystem crossing processes on ∼500 fs and 13 ns timescales, which were not observed in the TRXSS measurement, are likely to involve no structural change.
Extended Data Figure 10 Species-associated RDFs of the four structures obtained from the SVD and principal-component analyses (black) and their fits (red) obtained by using the Debye–Waller factor and the constraint of the symmetric structure for the S0 state.
For each state, the structural parameters obtained from the fits and their standard errors determined from 50 independent measurements are shown together. It can be seen that the structural parameters of S1, T1 and the tetramer obtained from the fits using the Debye–Waller factor and the symmetric constraint for S0 are similar to the values given in Fig. 2d.
Supplementary information
Supplementary Information
This file contains supplementary Text and Data. (PDF 176 kb)
Rights and permissions
About this article
Cite this article
Kim, K., Kim, J., Nozawa, S. et al. Direct observation of bond formation in solution with femtosecond X-ray scattering. Nature 518, 385–389 (2015). https://doi.org/10.1038/nature14163
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14163
This article is cited by
-
Observing the primary steps of ion solvation in helium droplets
Nature (2023)
-
Filming enhanced ionization in an ultrafast triatomic slingshot
Communications Chemistry (2023)
-
Melting domain size and recrystallization dynamics of ice revealed by time-resolved x-ray scattering
Nature Communications (2023)
-
Determining the charge distribution and the direction of bond cleavage with femtosecond anisotropic x-ray liquidography
Nature Communications (2022)
-
Ultrafast coherent motion and helix rearrangement of homodimeric hemoglobin visualized with femtosecond X-ray solution scattering
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.