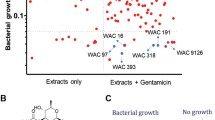
Abstract
The emergence and spread of carbapenem-resistant Gram-negative pathogens is a global public health problem. The acquisition of metallo-β-lactamases (MBLs) such as NDM-1 is a principle contributor to the emergence of carbapenem-resistant Gram-negative pathogens that threatens the use of penicillin, cephalosporin and carbapenem antibiotics to treat infections. To date, a clinical inhibitor of MBLs that could reverse resistance and re-sensitize resistant Gram-negative pathogens to carbapenems has not been found. Here we have identified a fungal natural product, aspergillomarasmine A (AMA), that is a rapid and potent inhibitor of the NDM-1 enzyme and another clinically relevant MBL, VIM-2. AMA also fully restored the activity of meropenem against Enterobacteriaceae, Acinetobacter spp. and Pseudomonas spp. possessing either VIM or NDM-type alleles. In mice infected with NDM-1-expressing Klebsiella pneumoniae, AMA efficiently restored meropenem activity, demonstrating that a combination of AMA and a carbapenem antibiotic has therapeutic potential to address the clinical challenge of MBL-positive carbapenem-resistant Gram-negative pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Frère, J. M. Beta-Lactamases (Nova Science, 2011)
Pitout, J. D. & Laupland, K. B. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8, 159–166 (2008)
Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States (Atlanta, Georgia, 2013)
Edelstein, M. V. et al. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect. Dis. 13, 867–876 (2013)
Patel, G. & Bonomo, R. A. “Stormy waters ahead”: global emergence of carbapenemases. Front. Microbiol. 4, 48 (2013)
Frias, M. et al. New Delhi metallo-β-lactamase-producing Escherichia coli associated with endoscopic retrograde cholangiopancreatography — Illinois, 2013. MMWR Morb. Mortal. Wkly Rep. 62, 1051 (2014)
Chang, Y. Laboratory Trends, December 17. 8 (BC Public Health Microbiology & Reference Laboratory, Vancouver, British Columbia, 2013)
Yigit, H. et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45, 1151–1161 (2001)
Bush, K. Proliferation and significance of clinically relevant β-lactamases. Ann. NY Acad. Sci. 1277, 84–90 (2013)
Drawz, S. M. & Bonomo, R. A. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 23, 160–201 (2010)
Fast, W. & Sutton, L. D. Metallo-β-lactamase: inhibitors and reporter substrates. Biochim. Biophys. Acta 1834, 1648–1659 (2013)
Buynak, J. D. β-Lactamase inhibitors: a review of the patent literature (2010–2013). Expert Opin. Ther. Pat. 23, 1469–1481 (2013)
Ricci, D. P. & Silhavy, T. J. The Bam machine: a molecular cooper. Biochim. Biophys. Acta 1818, 1067–1084 (2012)
Blair, J. M. & Piddock, L. J. Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: an update. Curr. Opin. Microbiol. 12, 512–519 (2009)
Haenni, A. L. et al. Structure chimique des aspergillomarasmines A et B. Helv. Chim. Acta 48, 729–750 (1965)
Mikami, Y. & Suzuki, T. Novel microbial inhibitors of angiotensin-converting enzyme, aspergillomarasmines A and B. Agric. Biol. Chem. 47, 2693–2695 (1983)
Arai, K. et al. Aspergillomarasmine A and B, potent microbial inhibitors of endothelin-converting enzyme. Biosci. Biotechnol. Biochem. 57, 1944–1945 (1993)
Matsuura, A. et al. Pharmacological profiles of aspergillomarasmines as endothelin converting enzyme inhibitors. Jpn. J. Pharmacol. 63, 187–193 (1993)
Hernandez Valladares, M. et al. Zn(II) dependence of the Aeromonas hydrophila AE036 metallo-β-lactamase activity and stability. Biochemistry 36, 11534–11541 (1997)
Docquier, J. D. et al. On functional and structural heterogeneity of VIM-type metallo-beta-lactamases. J. Antimicrob. Chemother. 51, 257–266 (2003)
Pillai, S. K., Moellering, R. C., Jr & Eliopoulos, G. M. in Antibiotics in Laboratory Medicine (ed. V. Lorian ) 365–440 (Williams & Wilkins, 2005)
Shlaes, D. M. New β-lactam-β-lactamase inhibitor combinations in clinical development. Ann. NY Acad. Sci. 1277, 105–114 (2013)
Holmquist, B., Bunning, P. & Riordan, J. F. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal. Biochem. 95, 540–548 (1979)
Lee, M., Hesek, D. & Mobashery, S. A practical synthesis of nitrocefin. J. Org. Chem. 70, 367–369 (2005)
Petersen, T. N., Brunak, S., von Heijne, G. & Nielsen, H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods 8, 785–786 (2011)
Zhang, J. H., Chung, T. D. & Oldenburg, K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999)
King, D. T., Worrall, L. J., Gruninger, R. & Strynadka, N. C. New Delhi metallo-β-lactamase: structural insights into β-lactam recognition and inhibition. J. Am. Chem. Soc. 134, 11362–11365 (2012)
EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0. (2014)
Marra, A. et al. Effect of linezolid on the 50% lethal dose and 50% protective dose in treatment of infections by Gram-negative pathogens in naive and immunosuppressed mice and on the efficacy of ciprofloxacin in an acute murine model of septicemia. Antimicrob. Agents Chemother. 56, 4671–4675 (2012)
Endimiani, A. et al. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55, 82–85 (2011
Acknowledgements
We thank M. Mulvey (Public Health Agency of Canada) and R. Melano (Public Health Ontario) for clinical strains. We thank L. Rossi for her work in constructing the screening strain. AMA inhibition activity on clinical strains by T.R.W. was funded by the UK Medical Research Council (G1100135). This research was funded by a Canadian Institutes of Health Research Grant (MT-13536), Natural Sciences and Engineering Research Council Grant (237480), and by a Canada Research Chair in Infectious Disease Pathogenesis (to B.K.C.) and Antibiotic Biochemistry (to G.D.W.).
Author information
Authors and Affiliations
Contributions
A.M.K., G.D.W., S.A.R.-Y., B.K.C., T.R.W. and N.C.S. designed experiments; G.D.P. and A.M.K. designed and engineered the E. coli strains and screened extracts; A.M.K. synthesized nitrocefin; A.M.K. cloned constructs; A.M.K. and D.T.K. purified enzymes; A.M.K. performed enzyme kinetics; W.W. and A.M.K. fermented WAC-138 and purified AMA; W.W. elucidated AMA structure; A.M.K. performed FIC experiments; D.T.K. performed ICP-MS; S.A.R.-Y. and B.K.C. designed the animal studies; S.A.R.-Y. and A.M.K. performed animal experiments; T.R.W. performed the clinical isolate screen; and A.M.K. and G.D.W. principally wrote the manuscript with input from all.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 6 IC50 inhibition profiles for select SBLs and ACE.
a, b, Experiments were done as in Fig. 1b for ACE and CTX-M-15 (black circles), KPC-2 (white circles), and TEM-1 (black squares). Error bars denote standard deviation of at least two replicates.
Extended Data Figure 7 Effects of meropenem dosage on spleen burden.
CD-1 mice were infected with K. pneumoniae N11-2218 by i.p. injection. Mice were treated with either PBS (n = 6) or various doses of meropenem (n = 3 per group) by s.c. injection. Mice were euthanized 48 h after infection, and the bacterial load in the spleen was determined by selective plating. Data are the means with standard error.
Rights and permissions
About this article
Cite this article
King, A., Reid-Yu, S., Wang, W. et al. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 510, 503–506 (2014). https://doi.org/10.1038/nature13445
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13445
This article is cited by
-
In-cell protein stability promotes antimicrobial resistance of metallo-β-lactamases
Nature Chemical Biology (2023)
-
Metformin reverse minocycline to inhibit minocycline-resistant Acinetobacter baumannii by destroy the outer membrane and enhance membrane potential in vitro
BMC Microbiology (2022)
-
A hydroxide lock for metallo-β-lactamases
Nature Chemistry (2022)
-
Scaffold size-dependent effect on the enhanced uptake of antibiotics and other compounds by Escherichia coli and Pseudomonas aeruginosa
Scientific Reports (2022)
-
Analysis of Antibacterial Action of Mammalian Host-Defense Cathelicidins and Induction of Resistance to Them in MβL-Producing Pseudomonas aeruginosa
Bulletin of Experimental Biology and Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.