Abstract
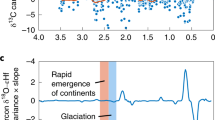
The observed stability of Earth’s climate over millions of years is thought to depend on the rate of carbon dioxide (CO2) release from the solid Earth being balanced by the rate of CO2 consumption by silicate weathering1. During the Cenozoic era, spanning approximately the past 66 million years, the concurrent increases in the marine isotopic ratios of strontium, osmium and lithium2,3,4 suggest that extensive uplift of mountain ranges may have stimulated CO2 consumption by silicate weathering5, but reconstructions of sea-floor spreading6 do not indicate a corresponding increase in CO2 inputs from volcanic degassing. The resulting imbalance would have depleted the atmosphere of all CO2 within a few million years7. As a result, reconciling Cenozoic isotopic records with the need for mass balance in the long-term carbon cycle has been a major and unresolved challenge in geochemistry and Earth history. Here we show that enhanced sulphide oxidation coupled to carbonate dissolution can provide a transient source of CO2 to Earth’s atmosphere that is relevant over geological timescales. Like drawdown by means of silicate weathering, this source is probably enhanced by tectonic uplift, and so may have contributed to the relative stability of the partial pressure of atmospheric CO2 during the Cenozoic. A variety of other hypotheses8,9,10 have been put forward to explain the ‘Cenozoic isotope-weathering paradox’, and the evolution of the carbon cycle probably depended on multiple processes. However, an important role for sulphide oxidation coupled to carbonate dissolution is consistent with records of radiogenic isotopes2,3, atmospheric CO2 partial pressure11,12 and the evolution of the Cenozoic sulphur cycle, and could be accounted for by geologically reasonable changes in the global dioxygen cycle, suggesting that this CO2 source should be considered a potentially important but as yet generally unrecognized component of the long-term carbon cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walker, J., Hays, P. & Kasting, J. A negative feedback mechanism for the long-term stabilization of the Earth’s surface temperature. J. Geophys. Res. 86, 9776–9782 (1981)
McArthur, J. M., Howarth, R. J. & Bailey, T. R. Strontium isotope stratigraphy: LOWESS version 3: best fit to the marine Sr-isotope curve for 0–509 Ma and accompanying look-up table for deriving numerical age. J. Geol. 109, 155–170 (2001)
Klemm, V., Levasseur, S., Frank, M., Hein, J. R. & Halliday, A. N. Osmium isotope stratigraphy of a marine ferromanganese crust. Earth Planet. Sci. Lett. 238, 42–48 (2005)
Misra, S. & Froelich, P. N. Lithium isotope history of Cenozoic seawater: changes in silicate weathering and reverse weathering. Science 335, 818–823 (2012)
Raymo, M., Ruddiman, W. & Froelich, P. Influence of late Cenozoic mountain building on ocean geochemical cycles. Geology 16, 649–653 (1988)
Müller, R. D., Sdrolias, M., Gaina, C., Steinberger, B. & Heine, C. Long-term sea-level fluctuations driven by ocean basin dynamics. Science 319, 1357–1362 (2008)
Berner, R. A. & Caldeira, K. The need for mass balance and feedback in the geochemical carbon cycle. Geology 25, 955 (1997)
Raymo, M. & Ruddiman, W. F. Tectonic forcing of late Cenozoic climate. Nature 359, 117–122 (1992)
Bickle, M. Metamorphic decarbonation, silicate weathering and the long-term carbon cycle. Terra Nova 8, 270–276 (1996)
Li, G. & Elderfield, H. Evolution of carbon cycle over the past 100 million years. Geochim. Cosmochim. Acta 103, 11–25 (2013)
Pagani, M., Zachos, J. C., Freeman, K. H., Tipple, B. & Bohaty, S. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science 309, 600–603 (2005)
Zhang, Y. G., Pagani, M., Liu, Z., Bohaty, S. M. & DeConto, R. A 40-million-year history of atmospheric CO2 . Phil. Trans. Royal. Soc. A 371, 1–20 (2013)
Galy, V., Beyssac, O., France-Lanord, C. & Eglinton, T. Recycling of graphite during Himalayan erosion: a geological stabilization of carbon in the crust. Science 322, 943–945 (2008)
Katz, M. et al. Biological overprint of the geological carbon cycle. Mar. Geol. 217, 323–338 (2005)
Kerrick, D. M. & Caldeira, K. Metamorphic CO2 degassing from orogenic belts. Chem. Geol. 145, 213–232 (1998)
Berner, K. E. & Berner, R. A. Global Environment: Water, Air and Geochemical Cycles 369–382 (Princeton Univ. Press, 2012)
Calmels, D., Gaillardet, J., Brenot, A. & France-Lanord, C. Sustained sulfide oxidation by physical erosion processes in the Mackenzie River basin: climatic perspectives. Geology 35, 1003 (2007)
Lerman, A., Wu, L. & Mackenzie, F. T. CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance. Mar. Chem. 106, 326–350 (2007)
Galy, A. & France-Lanord, C. Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem. Geol. 159, 31–60 (1999)
Calmels, D. et al. Contribution of deep groundwater to the weathering budget in a rapidly eroding mountain belt, Taiwan. Earth Planet. Sci. Lett. 303, 48–58 (2011)
Das, A., Chung, C.-H. & You, C.-F. Disproportionately high rates of sulfide oxidation from mountainous river basins of Taiwan orogeny: sulfur isotope evidence. Geophys. Res. Lett. 39, L12404 (2012)
Millot, R., Gaillardet, J., Dupre, B. & Allegre, C. Northern latitude chemical weathering rates: clues from the Mackenzie River Basin, Canada. Geochim. Cosmochim. Acta 67, 1305–1329 (2003)
Berner, R. A. et al. Isotope fractionation and atmospheric oxygen: Implications for Phanerozoic O2 evolution. Science 287, 1630–1633 (2000)
Georg, R. B., West, A. J., Vance, D., Newman, K. & Halliday, A. N. Is the marine osmium isotope record a probe for CO2 release from sedimentary rocks? Earth Planet. Sci. Lett. 367, 28–38 (2013)
Willenbring, J. K. & von Blanckenburg, F. Long-term stability of global erosion rates and weathering during late-Cenozoic cooling. Nature 465, 211–214 (2010)
Horita, J., Zimmermann, H. & Holland, H. D. Chemical evolution of seawater during the Phanerozoic: implications from the record of marine evaporites. Geochim. Cosmochim. Acta 66, 3733–3756 (2002)
Paytan, A., Kastner, M., Campbell, D. & Thiemens, M. H. Sulfur isotopic composition of Cenozoic seawater sulfate. Science 282, 1459–1462 (1998)
Watson, A. J., Lovelock, J. E. & Margulis, L. Methanogenesis, fires, and the regulation of atmospheric oxygen. Biosystems 10, 293–298 (1978)
Wildman, R. A. et al. Burning of forest materials under late Paleozoic high atmospheric oxygen levels. Geology 32, 457–460 (2004)
LaRowe, D. E. & Van Cappellen, P. Degradation of natural organic matter: A thermodynamic analysis. Geochim. Cosmochim. Acta 75, 2030–2042 (2011)
Kurtz, A. C., Kump, L. R., Arthur, M. A., Zachos, J. C. & Paytan, A. Early Cenozoic decoupling of the global carbon and sulfur cycles. Paleoceanography 18, 1090 (2003)
France-Lanord, C. & Derry, L. Organic carbon burial forcing of the carbon cycle from Himalayan erosion. Nature 390, 65–67 (1997)
Falkowski, P. G. et al. The rise of oxygen over the past 205 million years and the evolution of placental mammals. Science 309, 2202–2204 (2005)
Huh, Y., Birck, J.-L. & Allègre, C. J. Osmium isotope geochemistry in the Mackenzie River basin. Earth Planet. Sci. Lett. 222, 115–129 (2004)
Levasseur, S., Birck, J. & Allegre, C. The osmium riverine flux and the oceanic mass balance of osmium. Earth Planet. Sci. Lett. 174, 7–23 (1999)
Li, G., Ji, J., Chen, J. & Kemp, D. B. Evolution of the Cenozoic carbon cycle: the roles of tectonics and CO2 fertilization. Glob. Biogeochem. Cycles 23, GB1009 (2009)
Edmond, J. Himalayan tectonics, weathering processes, and the strontium isotope record in marine limestones. Science 258, 1594–1597 (1992)
Wu, N., Farquhar, J., Strauss, H., Kim, S.-T. & Canfield, D. E. Evaluating the S-isotope fractionation associated with Phanerozoic pyrite burial. Geochim. Cosmochim. Acta 74, 2053–2071 (2010)
Turchyn, A. V., Tipper, E. T., Galy, A., Lo, J.-K. & Bickle, M. J. Isotope evidence for secondary sulfide precipitation along the Marsyandi River, Nepal, Himalayas. Earth Planet. Sci. Lett. 374, 36–46 (2013)
Holser, W. T., Schidlowski, M., Mackenzie, F. T. & Maynard, J. B. in Chemical Cycles and the Evolution of Earth (eds Gergor, C. B., Garrels, R. M., Mackenzie, F. T., & Maynard, J. B. ) 105–173 (Wiley, 1988)
Gaillardet, J., Dupré, B., Louvat, P. & Allegre, C. J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 159, 3–30 (1999)
Habicht, K. S., Gade, M., Thamdrup, B., Berg, P. & Canfield, D. E. Calibration of sulfate levels in the Archean ocean. Science 298, 2372–2374 (2002)
Berner, R. Burial of organic carbon and pyrite sulfur in the modern ocean: its geochemical and environmental significance. Am. J. Sci. 282, 451–473 (1982)
Galy, V. et al. Efficient organic carbon burial in the Bengal fan sustained by the Himalayan erosional system. Nature 450, 407–410 (2007)
Meybeck, M. & Ragu, A. in Environment Information and Assessment 1–245 (UN Environment Programme, 1996)
Ivanov, M. V. in The Global Biogeochemical Sulphur Cycle (eds Ivanov, M. V. & Freney, J. R. ) 297–356 (Wiley, 1983)
Acknowledgements
Support for this work comes from a USC College Fellowship and a C-DEBI Graduate Fellowship to M.A.T., NSF funding (NSF-EAR/GLD-1053504 and EAR/GLTG-1227192) to A.J.W., and National Natural Science Foundation of China funding (grant nos 41173105, 41102103 and 41321062) to G.L. This is C-DEBI contribution #197.
Author information
Authors and Affiliations
Contributions
M.A.T. and A.J.W. designed the study, the conceptual model experiments and the sulphur isotope modelling. G.L. contributed the isotope mass balance model. M.A.T. and A.J.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Riverine input fluxes and calculated pyrite burial fluxes from 0 to 50 Myr ago for constant δ34S values of riverine input and Δpyrite-seawater.
Solid and dashed black lines indicate the total and pyrite-derived input fluxes of sulphate from the Li and Elderfield10 model, respectively. The green band indicates the range of calculated pyrite burial fluxes for experiments where the δ34S value of riverine S flux was equal to 10‰ and Δpyrite-seawater was varied between −30 and −50‰. The pink band indicates the range of calculated pyrite burial fluxes for experiments where the δ34S value of riverine S flux was equal to 5‰ and Δpyrite-seawater was varied between −30 and −50‰. The blue band indicates the range of calculated pyrite burial fluxes for experiments where the δ34S value of riverine was equal to 0‰ and Δpyrite-seawater was varied between −30 and −50‰. The khaki bar indicates the range of pyrite burial fluxes estimated from the pyrite content of marine sediments by ref. 40.
Extended Data Figure 2 Calculated Δpyrite-seawater values from 0 to 30 Myr ago for constant δ34S values of riverine input and pyrite burial fluxes.
The solid and dashed black lines indicate the average and minimum Δpyrite-seawater values for the Phanerozoic based on the isotopic offset between coeval marine sulphate and sulphide minerals38,45. The thickness of the solid line reflects 1 s.d. of the average Δpyrite-seawater from ref. 38. The green band indicates the range of calculated Δpyrite-seawater values for experiments where the pyrite burial flux was 1 × 1018 mol S Myr−1 and the δ34S value of riverine was varied between 0 and 10‰. The blue band indicates the range of calculated Δpyrite-seawater values for experiments where the pyrite burial flux was 0.67 × 1018 mol S Myr−1 and the δ34S value of riverine was varied between 0 and 10‰. The brown band indicates the range of calculated Δpyrite-seawater values for experiments where the pyrite burial flux was 0.22 × 1018 mol S Myr−1 and the δ34S value of riverine was varied between 0 and 10‰.
Extended Data Figure 3 Calculated δ34S values of riverine input from 0 to 30 Myr ago for constant Δpyrite-seawater values and pyrite burial fluxes.
The solid black line on the vertical axis indicates the range of estimates for the modern δ34S value of riverine input27,40. The green band indicates the range of calculated δ34S values of riverine input for experiments where the pyrite burial flux was 1 × 1018 mol S Myr−1 and Δpyrite-seawater was varied between −30 and −50‰. The blue band indicates the range of calculated δ34S values of riverine input for experiments where the pyrite burial flux was 0.67 × 1018 mol S Myr−1 and Δpyrite-seawater was varied between −30 and −50‰. The brown band indicates the range of calculated δ34S values of riverine input for experiments where the pyrite burial flux was 0.22 × 1018 mol S Myr−1 and Δpyrite-seawater was varied between −30 and −50‰.
Extended Data Figure 4 Integrated O2 consumption and CO2 production implied by the S cycle inverse model for different input parameters.
The O2 consumption values are presented in molar units. Importantly, the implied  values ignore other processes affecting the O2 budget (for example changes in the organic C cycle). Negative values of both CO2 and O2 consumption reflect net sulphide burial. Independent estimates of the δ34S of riverine input40,46 and the average Δpyrite-seawater (ref. 38) correspond to the parameter combinations that produce net sulphide oxidation (that is, positive values) and are marked with stars. a, The model results integrated from 0 to 50 Myr ago. b, The model results integrated from 0 to 15 Myr ago following the results of the Li and Elderfield10 model, which suggests that sulphide oxidation acted at least in part to balance enhanced CO2 consumption over this time period. The dashed horizontal lines represent the CO2 release required to compensate fully for a 10% (blue), 25% (purple) or 50% (orange) increase in continental silicate weathering that occurred linearly over the past 15 Myr assuming a modern flux of 3.5 × 1018 mol Myr−1 (Methods). The 25% increase corresponds to the increase predicted by the Li and Elderfield10 mass balance model.
values ignore other processes affecting the O2 budget (for example changes in the organic C cycle). Negative values of both CO2 and O2 consumption reflect net sulphide burial. Independent estimates of the δ34S of riverine input40,46 and the average Δpyrite-seawater (ref. 38) correspond to the parameter combinations that produce net sulphide oxidation (that is, positive values) and are marked with stars. a, The model results integrated from 0 to 50 Myr ago. b, The model results integrated from 0 to 15 Myr ago following the results of the Li and Elderfield10 model, which suggests that sulphide oxidation acted at least in part to balance enhanced CO2 consumption over this time period. The dashed horizontal lines represent the CO2 release required to compensate fully for a 10% (blue), 25% (purple) or 50% (orange) increase in continental silicate weathering that occurred linearly over the past 15 Myr assuming a modern flux of 3.5 × 1018 mol Myr−1 (Methods). The 25% increase corresponds to the increase predicted by the Li and Elderfield10 mass balance model.
Rights and permissions
About this article
Cite this article
Torres, M., West, A. & Li, G. Sulphide oxidation and carbonate dissolution as a source of CO2 over geological timescales. Nature 507, 346–349 (2014). https://doi.org/10.1038/nature13030
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13030
This article is cited by
-
Deep CO2 release and the carbon budget of the central Apennines modulated by geodynamics
Nature Geoscience (2024)
-
Ecotoxic effect in Allium cepa due to sphalerite weathering arising in calcareous conditions
Environmental Geochemistry and Health (2024)
-
Neogene burial of organic carbon in the global ocean
Nature (2023)
-
Enhanced clay formation key in sustaining the Middle Eocene Climatic Optimum
Nature Geoscience (2023)
-
Integrating terrestrial and aquatic ecosystems to constrain estimates of land-atmosphere carbon exchange
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.