Abstract
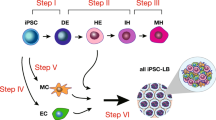
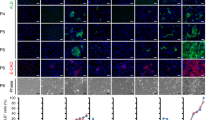
A critical shortage of donor organs for treating end-stage organ failure highlights the urgent need for generating organs from human induced pluripotent stem cells (iPSCs)1. Despite many reports describing functional cell differentiation2,3,4, no studies have succeeded in generating a three-dimensional vascularized organ such as liver. Here we show the generation of vascularized and functional human liver from human iPSCs by transplantation of liver buds created in vitro (iPSC-LBs). Specified hepatic cells (immature endodermal cells destined to track the hepatic cell fate) self-organized into three-dimensional iPSC-LBs by recapitulating organogenetic interactions between endothelial and mesenchymal cells5. Immunostaining and gene-expression analyses revealed a resemblance between in vitro grown iPSC-LBs and in vivo liver buds. Human vasculatures in iPSC-LB transplants became functional by connecting to the host vessels within 48 hours. The formation of functional vasculatures stimulated the maturation of iPSC-LBs into tissue resembling the adult liver. Highly metabolic iPSC-derived tissue performed liver-specific functions such as protein production and human-specific drug metabolism without recipient liver replacement6. Furthermore, mesenteric transplantation of iPSC-LBs rescued the drug-induced lethal liver failure model. To our knowledge, this is the first report demonstrating the generation of a functional human organ from pluripotent stem cells. Although efforts must ensue to translate these techniques to treatments for patients, this proof-of-concept demonstration of organ-bud transplantation provides a promising new approach to study regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Klein, A. S. et al. Organ donation and utilization in the United States, 1999–2008. Am. J. Transplant. 10, 973–986 (2010)
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547–551 (2011)
Kroon, E. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature Biotechnol. 26, 443–452 (2008)
Si-Tayeb, K. et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297–305 (2010)
Matsumoto, K., Yoshitomi, H., Rossant, J. & Zaret, K. S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294, 559–563 (2001)
Espejel, S. et al. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J. Clin. Invest. 120, 3120–3126 (2010)
Kobayashi, T. et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142, 787–799 (2010)
Zhao, R. & Duncan, S. A. Embryonic development of the liver. Hepatology 41, 956–967 (2005)
Si-Tayeb, K., Lemaigre, F. P. & Duncan, S. A. Organogenesis and development of the liver. Dev. Cell 18, 175–189 (2010)
Gouysse, G. et al. Relationship between vascular development and vascular differentiation during liver organogenesis in humans. J. Hepatol. 37, 730–740 (2002)
Jung, J., Zheng, M., Goldfarb, M. & Zaret, K. S. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 284, 1998–2003 (1999)
Korzh, S. et al. Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev. Biol. 8, 84 (2008)
Koike, N. et al. Tissue engineering: creation of long-lasting blood vessels. Nature 428, 138–139 (2004)
Takebe, T. et al. Generation of functional human vascular network. Transplant. Proc. 44, 1130–1133 (2012)
Ding, B. S. et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468, 310–315 (2010)
Martinez-Hernandez, A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab. Invest. 51, 57–74 (1984)
Ishizaki, T. et al. Pharmacokinetics of ketoprofen following single oral, intramuscular and rectal doses and after repeated oral administration. Eur. J. Clin. Pharmacol. 18, 407–414 (1980)
Yu, A. M., Idle, J. R. & Gonzalez, F. J. Polymorphic cytochrome P450 2D6: humanized mouse model and endogenous substrates. Drug Metab. Rev. 36, 243–277 (2004)
Katoh, M. et al. In vivo drug metabolism model for human cytochrome P450 enzyme using chimeric mice with humanized liver. J. Pharm. Sci. 96, 428–437 (2007)
Kamimura, H. et al. Assessment of chimeric mice with humanized liver as a tool for predicting circulating human metabolites. Drug Metab. Pharmacokinet. 25, 223–235 (2010)
Hasegawa, M. et al. The reconstituted 'humanized liver' in TK-NOG mice is mature and functional. Biochem. Biophys. Res. Commun. 405, 405–410 (2011)
Dudley, S. C., Jr Beware of cells bearing gifts: cell replacement therapy and arrhythmic risk. Circ. Res. 97, 99–101 (2005)
Kobayashi, S. et al. Reconstruction of human elastic cartilage by a CD44+CD90+ stem cell in the ear perichondrium. Proc. Natl Acad. Sci. USA 108, 14479–14484 (2011)
Acknowledgements
We thank F. Kawamata, E. Yoshizawa, Y. Suzuki, S. Nakai, Y. Takahashi, N. Tsuchida and N. Sasaki for kindly providing technical support; J. Nakabayashi, K. Yasumura, R. Fujiwara, T. Amiya, A. Nakano, Y. Mitsuhashi and all of the members of our laboratory for help with several experiments and comments. We are also grateful to D. Fukumura, Y. Goshima, T. Hirose, M. Ichino, U. Yokoyama, T. Ogawa and R. K. Jain for critical evaluation of the manuscript. This work was supported by grants to H. Taniguchi from the Strategic Promotion of Innovative Research and Development (S-innovation, 62890004) of the Japan Science and Technology Agency (JST). This work was also supported by the Grants-in-Aid of the Ministry of Education, Culture, Sports, Science, and Technology of Japan to T. Takebe (no. 24106510, 24689052), N. Koike (no. 22390260) and H. Taniguchi (no. 21249071, 25253079); by the Specified Research Grant of the Takeda Science Foundation and a grant from the Japan IDDM network to H. Taniguchi; and by a grant of the Yokohama Foundation for Advanced Medical Science to T. Takebe.
Author information
Authors and Affiliations
Contributions
T.T. conceived the study, performed the experiments, collected and analysed the data and drafted the manuscript. K.S., M.E., H.K., M.K., T.O., R-R.Z. and S.A. performed the experiments with the technical guidance and expertise of K.S., Y.-W.Z., Y.U. and T.T. K.S., Y.-W.Z., N.K., Y.A. and H.T. provided critical advice on the research strategy and design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-20, Supplementary Methods, Supplementary Tables 1-3, Supplementary Discussion and Supplementary References. This file was replaced on 27 November 2013 to include the Supplementary Discussion and Tables. (PDF 9046 kb)
Generation of human induced pluripotent stem cell-derived liver bud by recapitulating organogenesis
This video shows formation of human induced pluripotent stem cell-derived liver bud by recapitulating organogenetic interactions. (MOV 7371 kb)
Formation of functional human vascular networks inside the hiPSC-LB transplant
This video shows the z stack images of patent human vasculatures inside the hiPSC-LB transplants at day 4. (MOV 3646 kb)
Successful engraftment of hiPSC-derived hepatic cells through the functional vessel formation
This video shows the successful engraftment of hiPSC-Heps along with the functional vascular networks at day 4. (MOV 3968 kb)
3D visualization of the direct connection among human and host vessels by whole mount immunostaining
This video shows the connection among the human and host vessels visualised by immunostaining of the explants. Volocity software was used to reconstruct 3D image. (MOV 5222 kb)
Rights and permissions
About this article
Cite this article
Takebe, T., Sekine, K., Enomura, M. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013). https://doi.org/10.1038/nature12271
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12271
This article is cited by
-
Human intestinal organoid-derived PDGFRα + mesenchymal stroma enables proliferation and maintenance of LGR4 + epithelial stem cells
Stem Cell Research & Therapy (2024)
-
Intraocular liver spheroids for non-invasive high-resolution in vivo monitoring of liver cell function
Nature Communications (2024)
-
Three Dimensional Bioprinting for Hepatic Tissue Engineering: From In Vitro Models to Clinical Applications
Tissue Engineering and Regenerative Medicine (2024)
-
Comparative transcriptomic and phenotypic analysis of induced pluripotent stem cell hepatocyte-like cells and primary human hepatocytes
Cell and Tissue Research (2024)
-
Infantile hemangioma models: is the needle in a haystack?
Journal of Translational Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.