Abstract
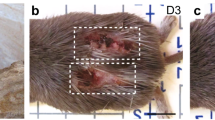
Evolutionary modification has produced a spectrum of animal defence traits to escape predation, including the ability to autotomize body parts to elude capture1,2. After autotomy, the missing part is either replaced through regeneration (for example, in urodeles, lizards, arthropods and crustaceans) or permanently lost (such as in mammals). Although most autotomy involves the loss of appendages (legs, chelipeds, antennae or tails, for example), skin autotomy can occur in certain taxa of scincid and gekkonid lizards3. Here we report the first demonstration of skin autotomy in Mammalia (African spiny mice, Acomys). Mechanical testing showed a propensity for skin to tear under very low tension and the absence of a fracture plane. After skin loss, rapid wound contraction was followed by hair follicle regeneration in dorsal skin wounds. Notably, we found that regenerative capacity in Acomys was extended to ear holes, where the mice exhibited complete regeneration of hair follicles, sebaceous glands, dermis and cartilage. Salamanders capable of limb regeneration form a blastema (a mass of lineage-restricted progenitor cells4) after limb loss, and our findings suggest that ear tissue regeneration in Acomys may proceed through the assembly of a similar structure. This study underscores the importance of investigating regenerative phenomena outside of conventional model organisms, and suggests that mammals may retain a higher capacity for regeneration than was previously believed. As re-emergent interest in regenerative medicine seeks to isolate molecular pathways controlling tissue regeneration in mammals, Acomys may prove useful in identifying mechanisms to promote regeneration in lieu of fibrosis and scarring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Maginnis, T. L. The costs of autotomy and regeneration in animals: a review and framework for future research. Behav. Ecol. 17, 857–872 (2006)
Shargal, E., Rath-Wolfson, L., Kronfeld, N. & Dayan, T. Ecological and histological aspects of tail loss in spiny mice (Rodentia: Muridae, Acomys) with a review of its occurrence in rodents. J. Zool. 249, 187–193 (1999)
Bauer, A. M., Russell, A. P. & Shadwick, R. E. Mechanical properties and morphological correlates of fragile skin in gekkonid lizards. J. Exp. Biol. 145, 79–102 (1989)
Kragl, M. et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 460, 60–65 (2009)
Dubost, G. & Gasc, J.-P. The process of total tail autotomy in the South-American rodent, Proechimys . J. Zool. 212, 563–572 (1987)
Vogel, H. G. Correlation between tensile-strength and collagen content in rat skin — effect of age and cortisol treatment. Connect. Tissue Res. 2, 177–182 (1974)
Seifert, A. W., Monaghan, J. R., Voss, S. R. & Maden, M. Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS One 7, e32875 (2012)
Dang, C. M. et al. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast. Reconstr. Surg. 111, 2273–2285 (2003)
Soo, C. et al. Differential expression of matrix metalloproteinases and their tissue-derived inhibitors in cutaneous wound repair. Plast. Reconstr. Surg. 105, 638–647 (2000)
Yannas, I. V. Tissue and Organ Regeneration in Adults (Springer, 2001)
McGowan, K. M. & Coulombe, P. A. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J. Cell Biol. 143, 469–486 (1998)
Andl, T., Reddy, S. T., Gaddapara, T. & Millar, S. E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, 643–653 (2002)
DasGupta, R. & Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 (1999)
Botchkarev, V. A. & Sharov, A. A. BMP signaling in the control of skin development and hair follicle growth. Differentiation 72, 512–526 (2004)
Driskell, R. R., Giangreco, A., Jensen, K. B., Mulder, K. W. & Watt, F. M. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 136, 2815–2823 (2009)
Billingham, R. E. & Russell, P. S. Incomplete wound contracture and the phenomenon of hair neogenesis in rabbits’ skin. Nature 177, 791–792 (1956)
Breedis, C. Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer Res. 14, 575–579 (1954)
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007)
Vorontsova, M. A. & Liozner, L. D. Asexual propagation and Regeneration 377–379 (Pergamon, 1960)
Borgens, R. B. Mice regrow the tips of their foretoes. Science 217, 747–750 (1982)
Muneoka, K., Allan, C. H., Yang, X., Lee, J. & Han, M. Mammalian regeneration and regenerative medicine. Birth Defects Res. C Embryo Today 84, 265–280 (2008)
Clark, L. D., Clark, R. K. & Heber-Katz, E. A new murine model for mammalian wound repair and regeneration. Clin. Immunol. Immunopathol. 88, 35–45 (1998)
Chalkley, D. T. A quantitative histological analysis of forelimb regeneration in Triturus viridescens . J. Morphol. 94, 21–70 (1954)
Globus, M., Vethamany-Globus, S. & Lee, Y. C. Effect of apical epidermal cap on mitotic cycle and cartilage differentiation in regeneration blastemata in the newt, Notophthalmus viridescens . Dev. Biol. 75, 358–372 (1980)
Neufeld, D. A. & Day, F. A. Perspective: a suggested role for basement membrane structures during newt limb regeneration. Anat. Rec. 246, 155–161 (1996)
Calve, S., Odelberg, S. J. & Simon, H. G. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev. Biol. 344, 259–271 (2010)
Satoh, A., Makanae, A., Hirata, A. & Satou, Y. Blastema induction in aneurogenic state and Prrx-1 regulation by MMPs and FGFs in Ambystoma mexicanum limb regeneration. Dev. Biol 355, 263–274 (2011)
Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 127, 526–537 (2007)
Gundersen, H. J. G. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J. Microsc. 111, 219–223 (1977)
Kiama, S. G., Maina, J. N., Bhattacharjee, J. & Weyrauch, K. D. Functional morphology of the pecten oculi in the nocturnal spotted eagle owl (Bubo bubo africanus), and the diurnal black kite (Milvus migrans) and domestic fowl (Gallus gallus var. domesticus): a comparative study. J. Zool. 254, 521–528 (2001)
Weibel, E. R. Stereological Methods (Academic, 1979)
Acknowledgements
We thank John Kahiro for assisting during materials testing and the Department of Mechanical Engineering, University of Nairobi, for use of their equipment. We thank John Kimani, Stanley Marete and Jackson Mugweru, for help with animal care and materials procurement in Nairobi, Ekiru Ekaran for field assistance, and Bernard Agwanda, Darcy Ogada, and Hillary Young for help with identification and natural history of Acomys. Conversations with Steve Takata and Truman Young drew our attention to this phenomenon.
Author information
Authors and Affiliations
Contributions
A.W.S., J.R.G., T.M.P. and M.M. formulated the research. A.W.S, M.G.S, M.M. and S.G.K., performed the research and analysed the data. A.W.S. wrote the manuscript and all authors discussed the results, commented on and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10 and Supplementary References. (PDF 28697 kb)
Rights and permissions
About this article
Cite this article
Seifert, A., Kiama, S., Seifert, M. et al. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489, 561–565 (2012). https://doi.org/10.1038/nature11499
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11499
This article is cited by
-
The efficacy of adipose-derived stem cells in burn injuries: a systematic review
Cellular & Molecular Biology Letters (2024)
-
Animal models to study cardiac regeneration
Nature Reviews Cardiology (2024)
-
Biophysical control of plasticity and patterning in regeneration and cancer
Cellular and Molecular Life Sciences (2024)
-
Evolutionary dynamics of whole-body regeneration across planarian flatworms
Nature Ecology & Evolution (2023)
-
Comparative analysis of Acomys cahirinus and Mus musculus responses to genotoxicity, oxidative stress, and inflammation
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.