Abstract
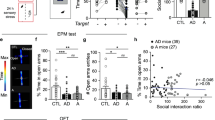
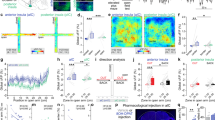
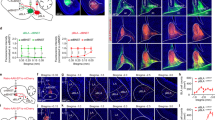
Anxiety—a sustained state of heightened apprehension in the absence of immediate threat—becomes severely debilitating in disease states1. Anxiety disorders represent the most common of psychiatric diseases (28% lifetime prevalence)2 and contribute to the aetiology of major depression and substance abuse3,4. Although it has been proposed that the amygdala, a brain region important for emotional processing5,6,7,8, has a role in anxiety9,10,11,12,13, the neural mechanisms that control anxiety remain unclear. Here we explore the neural circuits underlying anxiety-related behaviours by using optogenetics with two-photon microscopy, anxiety assays in freely moving mice, and electrophysiology. With the capability of optogenetics14,15,16 to control not only cell types but also specific connections between cells, we observed that temporally precise optogenetic stimulation of basolateral amygdala (BLA) terminals in the central nucleus of the amygdala (CeA)—achieved by viral transduction of the BLA with a codon-optimized channelrhodopsin followed by restricted illumination in the downstream CeA—exerted an acute, reversible anxiolytic effect. Conversely, selective optogenetic inhibition of the same projection with a third-generation halorhodopsin15 (eNpHR3.0) increased anxiety-related behaviours. Importantly, these effects were not observed with direct optogenetic control of BLA somata, possibly owing to recruitment of antagonistic downstream structures. Together, these results implicate specific BLA–CeA projections as critical circuit elements for acute anxiety control in the mammalian brain, and demonstrate the importance of optogenetically targeting defined projections, beyond simply targeting cell types, in the study of circuit function relevant to neuropsychiatric disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lieb, R. Anxiety disorders: clinical presentation and epidemiology. Handb. Exp. Pharmacol. 169, 405–432 (2005)
Kessler, R. C. et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005)
Koob, G. F. Brain stress systems in the amygdala and addiction. Brain Res. 1293, 61–75 (2009)
Ressler, K. J. & Mayberg, H. S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neurosci. 10, 1116–1124 (2007)
LeDoux, J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 23, 727–738 (2003)
Pare, D., Quirk, G. J. & Ledoux, J. E. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 92, 1–9 (2004)
Tye, K. M., Stuber, G. D., de Ridder, B., Bonci, A. & Janak, P. H. Rapid strengthening of thalamo-amygdala synapses mediates cue–reward learning. Nature 453, 1253–1257 (2008)
Weiskrantz, L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J. Comp. Physiol. Psychol. 49, 381–391 (1956)
Kalin, N. H., Shelton, S. E. & Davidson, R. J. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 24, 5506–5515 (2004)
Lesscher, H. M. et al. Amygdala protein kinase C epsilon regulates corticotropin-releasing factor and anxiety-like behavior. Genes Brain Behav. 7, 323–333 (2008)
Etkin, A., Prater, K. E., Schatzberg, A. F., Menon, V. & Greicius, M. D. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry 66, 1361–1372 (2009)
Lyons, A. M. & Thiele, T. E. Neuropeptide Y conjugated to saporin alters anxiety-like behavior when injected into the central nucleus of the amygdala or basomedial hypothalamus in BALB/cJ mice. Peptides 31, 2193–2199 (2010)
Roozendaal, B., McEwen, B. S. & Chattarji, S. Stress, memory and the amygdala. Nature Rev. Neurosci. 10, 423–433 (2009)
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neurosci. 8, 1263–1268 (2005)
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010)
Deisseroth, K. Optogenetics: controlling the brain with light. Sci. Am. 303, 48–55 (2010)
Woods, J. H., Katz, J. L. & Winger, G. Benzodiazepines: use, abuse, and consequences. Pharmacol. Rev. 44, 151–347 (1992)
Ciocchi, S. et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010)
Haubensak, W. et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276 (2010)
Carlsen, J. Immunocytochemical localization of glutamate decarboxylase in the rat basolateral amygdaloid nucleus, with special reference to GABAergic innervation of amygdalostriatal projection neurons. J. Comp. Neurol. 273, 513–526 (1988)
Smith, Y. & Pare, D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J. Comp. Neurol. 342, 232–248 (1994)
McDonald, A. J. Cytoarchitecture of the central amygdaloid nucleus of the rat. J. Comp. Neurol. 208, 401–418 (1982)
Krettek, J. E. & Price, J. L. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J. Comp. Neurol. 178, 255–279 (1978)
Krettek, J. E. & Price, J. L. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J. Comp. Neurol. 178, 225–253 (1978)
Davis, M. in The Amygdala: A Functional Analysis (ed. Aggleton, J. P. ) 213–288 (Oxford Univ. Press, 2000)
Pitkanen, A. in The Amygdala: A Functional Analysis (ed. Aggleton, J. P. ) 31–99 (Oxford Univ. Press, 2000)
Carola, V., D’Olimpio, F., Brunamonti, E., Mangia, F. & Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 134, 49–57 (2002)
Davis, M., Walker, D. L., Miles, L. & Grillon, C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135 (2010)
Shin, L. M. & Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191 (2010)
Gray, J. A. & McNaughton, N. The neuropsychology of anxiety: reprise. Nebr. Symp. Motiv. 43, 61–134 (1996)
Acknowledgements
We would like to thank P. Janak, H. Fields, G. Stuber, E. Thomas, F. Zhang, I. Witten, V. Sohal, T. Davidson and M. Warden as well as J. Mattis, R. Durand, M. Mogri, J. Mirzabekov and E. Steinberg for discussions, and the entire K.D. laboratory for their support. All viruses were packaged at University of North Carolina (UNC) Vector Core. Supported by NIMH (1F32MH088010-01, K.M.T.), NARSAD (K.R.T.), Samsung Scholarship (S.-Y.K.), NSF IGERT Award 0801700 (L.G.) and the Defense Advanced Research Projects Agency Reorganization and Plasticity to Accelerate Injury Recovery (N66001-10-C-2010), the Alice Woo, Albert Yu, Snyder, and McKnight Foundations, as well as NIDA, NIMH and the NIH Pioneer Award (K.D.)
Author information
Authors and Affiliations
Contributions
K.M.T., R.P., S.-Y.K., L.E.F. and K.D. contributed to study design and data interpretation. K.M.T., R.P., S.-Y.K. and L.E.F. contributed to data collection and K.M.T. coordinated data collection and analysis. K.M.T., S.-Y.K., H.Z. and K.R.T. contributed to immunohistochemical processing, fluorescence imaging and quantitative analyses. K.M.T. and L.G. performed the behavioural and ex vivo electrophysiology statistical analyses. V.G. and C.R. contributed to the design of eNpHR3.0. C.R. cloned all constructs and managed viral packaging processes. K.D. supervised all aspects of the work. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-15 with legends, Supplementary Materials and Methods and additional references. (PDF 11353 kb)
Supplementary Movie 1
This movie shows a representative mouse from the ChR2:BLA-CeA group on elevated plus maze. The 15-minute elevated plus maze session is shown at 5x speed; each 5-min epoch is shown in 1 min and the duration of the light epoch is indicated by the appearance of blue text detailing light stimulation parameters. During the light-on epoch, the mouse increased open arm entry and open arm time. (MP4 6635 kb)
Rights and permissions
About this article
Cite this article
Tye, K., Prakash, R., Kim, SY. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011). https://doi.org/10.1038/nature09820
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09820
This article is cited by
-
Direct paraventricular thalamus-basolateral amygdala circuit modulates neuropathic pain and emotional anxiety
Neuropsychopharmacology (2024)
-
Identification of a novel perifornical-hypothalamic-area-projecting serotonergic system that inhibits innate panic and conditioned fear responses
Translational Psychiatry (2024)
-
Behavioral analysis of kainate receptor KO mice and the role of GluK3 subunit in anxiety
Scientific Reports (2024)
-
Basolateral amygdala neuropeptide Y system modulates binge ethanol consumption
Neuropsychopharmacology (2024)
-
Inhibition of fatty acid binding protein-5 in the basolateral amygdala induces anxiolytic effects and accelerates fear memory extinction
Psychopharmacology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.