Abstract
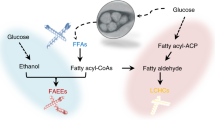
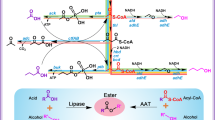
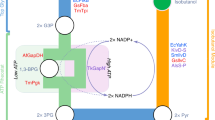
Increasing energy costs and environmental concerns have emphasized the need to produce sustainable renewable fuels and chemicals1. Major efforts to this end are focused on the microbial production of high-energy fuels by cost-effective ‘consolidated bioprocesses’2. Fatty acids are composed of long alkyl chains and represent nature’s ‘petroleum’, being a primary metabolite used by cells for both chemical and energy storage functions. These energy-rich molecules are today isolated from plant and animal oils for a diverse set of products ranging from fuels to oleochemicals. A more scalable, controllable and economic route to this important class of chemicals would be through the microbial conversion of renewable feedstocks, such as biomass-derived carbohydrates. Here we demonstrate the engineering of Escherichia coli to produce structurally tailored fatty esters (biodiesel), fatty alcohols, and waxes directly from simple sugars. Furthermore, we show engineering of the biodiesel-producing cells to express hemicellulases, a step towards producing these compounds directly from hemicellulose, a major component of plant-derived biomass.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fortman, J. L. et al. Biofuel alternatives to ethanol: pumping the microbial well. Trends Biotechnol. 26, 375–381 (2008)
Lynd, L. R., van Zyl, W. H., McBride, J. E. & Laser, M. Consolidated bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16, 577–583 (2005)
Hill, J., Nelson, E., Tilman, D., Polasky, S. & Tiffany, D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl Acad. Sci. USA 103, 11206–11210 (2006)
Rude, M. A. & Schirmer, A. New microbial fuels: a biotech perspective. Curr. Opin. Microbiol. 12, 274–281 (2009)
Magnuson, K., Jackowski, S., Rock, C. O. & Cronan, J. E. Regulation of fatty acid biosynthesis in Escherichia coli . Microbiol. Rev. 57, 522–542 (1993)
Jiang, P. & Cronan, J. E. Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J. Bacteriol. 176, 2814–2821 (1994)
Cho, H. & Cronan, J. E. Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis. J. Biol. Chem. 270, 4216–4219 (1995)
Lu, X., Vora, H. & Khosla, C. Overproduction of free fatty acids in E. coli: implications for biodiesel production. Metab. Eng. 10, 333–339 (2008)
Dehesh, K., Jones, A., Knutzon, D. S. & Voelker, T. A. Production of high levels of 8:0 and 10:0 fatty acids in transgenic canola by overexpression of Ch FatB2, a thioesterase cDNA from Cuphea hookeriana . Plant J. 9, 167–172 (1996)
Atsumi, S., Hanai, T. & Liao, J. C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451, 86–89 (2008)
Steen, E. J. et al. Metabolic engineering of Saccharomyces cerevisiae for the production of n-butanol. Microb. Cell Fact. 7, 36 (2008)
Oil. Market Report, International Energy Agency 〈http://omrpublic.iea.org/omrarchive/16jan08full.pdf〉 (16 January 2008)
Kalscheuer, R., Stolting, T. & Steinbuchel, A. Microdiesel: Escherichia coli engineered for fuel production. Microbiology 152, 2529–2536 (2006)
Rupilius, W. & Ahmad, S. The changing world of oleochemicals. Palm Oil Developments 44, 15–28 (2006)
Cheng, J. B. & Russell, D. W. Mammalian wax biosynthesis. I. Identification of two fatty acyl-Coenzyme A reductases with different substrate specificities and tissue distributions. J. Biol. Chem. 279, 37789–37797 (2004)
Metz, J. G., Pollard, M. R., Anderson, L., Hayes, T. R. & Lassner, M. W. Purification of a jojoba embryo fatty acyl-coenzyme A reductase and expression of its cDNA in high erucic acid rapeseed. Plant Physiol. 122, 635–644 (2000)
Reiser, S. & Somerville, C. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme A reductase. J. Bacteriol. 179, 2969–2975 (1997)
Knothe, G. “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuels 22, 1358–1364 (2008)
Ingram, L. O., Conway, T., Clark, D. P., Sewell, G. W. & Preston, J. F. Genetic engineering of ethanol production in Escherichia coli . Appl. Environ. Microbiol. 53, 2420–2425 (1987)
Adelsberger, H., Hertel, C., Glawischnig, E., Zverlov, V. V. & Schwarz, W. H. Enzyme system of Clostridium stercorarium for hydrolysis of arabinoxylan: reconstitution of the in vivo system from recombinant enzymes. Microbiology 150, 2257–2266 (2004)
Whitehead, T. R. & Hespell, R. B. The genes for three xylan-degrading activities from Bacteroides ovatus are clustered in a 3.8-kilobase region. J. Bacteriol. 172, 2408–2412 (1990)
Qian, Z. G., Xia, X. X., Choi, J. H. & Lee, S. Y. Proteome-based identification of fusion partner for high-level extracellular production of recombinant proteins in Escherichia coli . Biotechnol. Bioeng. 101, 587–601 (2008)
Tsuruta, H. et al. High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli . PLoS One 4, e4489 (2009)
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000)
Link, A. J., Phillips, D. & Church, G. M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179, 6228–6237 (1997)
Hoover, D. M. & Lubkowski, J. DNAWorks: an automated method for designing oligonucleotides for PCR-based gene synthesis. Nucleic Acids Res. 30, e43 (2002)
Li, M. Z. & Elledge, S. J. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nature Methods 4, 251–256 (2007)
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 1989)
Aldai, N., Osoro, K., Barron, L. J. & Najera, A. I. Gas–liquid chromatographic method for analysing complex mixtures of fatty acids including conjugated linoleic acids (cis9trans11 and trans10cis12 isomers) and long-chain (n-3 or n-6) polyunsaturated fatty acids: application to the intramuscular fat of beef meat. J. Chromatogr. A 1110, 133–139 (2006)
Acknowledgements
E.J.S. was supported by the Tien Scholar Environmental Fellowship and the Synthetic Biology Engineering Research Center (SynBERC). Y.K. and G.B. were supported by a grant from LS9, Inc. (South San Francisco, California) through the University of California Discovery Grant program. This research was performed at the Joint BioEnergy Institute. We thank M. Rude with help on the manuscript and J. Cronan and the LS9 Scientific Advisory Board for technical insight and discussion.
Author Contributions E.J.S., Y.K., G.B., Z.H., A.S., A.M., S.B.d.C. and J.D.K. conceived of the experiments. E.J.S. and Y.K. constructed the strains and metabolic pathways for fatty-acid-derived products and performed the production experiments. LS9 engineered and evaluated FAEE and fatty alcohol producing strains for thioesterase evaluations. G.B. conceived, constructed and performed the xylan-metabolizing pathway growth experiments. E.J.S. and Y.K. constructed the xylan-metabolizing, fatty acid production strain and performed the production experiments. E.J.S., Y.K., A.S., S.B.d.C. and J.D.K. drafted the manuscript. All authors approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
J.D.K. has financial interests in Amyris and LS9, both of which are involved in producing advanced biofuels. Z.H., A.S., A.M. and S.B.d.C. have a financial interest in LS9.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-2, Supplementary Figures 1-3 with Legends and Supplementary References. (PDF 408 kb)
Rights and permissions
About this article
Cite this article
Steen, E., Kang, Y., Bokinsky, G. et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463, 559–562 (2010). https://doi.org/10.1038/nature08721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08721
This article is cited by
-
Fruit residues as substrates for single-cell oil production by Rhodococcus species: physiology and genomics of carbohydrate catabolism
World Journal of Microbiology and Biotechnology (2024)
-
Bioprocessing of fermentable sugars derived from water hyacinth into microbial lipids and single cell proteins by oleaginous yeast Rhodosporidium toruloides NCIM 3547
Biomass Conversion and Biorefinery (2023)
-
A review on catalytic conversion of lignin into high-value chemicals over Ni-based catalysts
Biomass Conversion and Biorefinery (2023)
-
Escherichia coli coculture for de novo production of esters derived of methyl-branched alcohols and multi-methyl branched fatty acids
Microbial Cell Factories (2022)
-
A unique self-truncation of bacterial GH5 endoglucanases leads to enhanced activity and thermostability
BMC Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.