Abstract
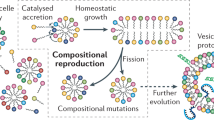
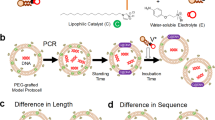
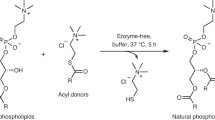
Contemporary phospholipid-based cell membranes are formidable barriers to the uptake of polar and charged molecules ranging from metal ions to complex nutrients. Modern cells therefore require sophisticated protein channels and pumps to mediate the exchange of molecules with their environment. The strong barrier function of membranes has made it difficult to understand the origin of cellular life and has been thought to preclude a heterotrophic lifestyle for primitive cells. Although nucleotides can cross dimyristoyl phosphatidylcholine membranes through defects formed at the gel-to-liquid transition temperature1,2, phospholipid membranes lack the dynamic properties required for membrane growth. Fatty acids and their corresponding alcohols and glycerol monoesters are attractive candidates for the components of protocell membranes because they are simple amphiphiles that form bilayer membrane vesicles3,4,5 that retain encapsulated oligonucleotides3,6 and are capable of growth and division7,8,9. Here we show that such membranes allow the passage of charged molecules such as nucleotides, so that activated nucleotides added to the outside of a model protocell spontaneously cross the membrane and take part in efficient template copying in the protocell interior. The permeability properties of prebiotically plausible membranes suggest that primitive protocells could have acquired complex nutrients from their environment in the absence of any macromolecular transport machinery; that is, they could have been obligate heterotrophs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chakrabarti, A. C., Breaker, R. R., Joyce, G. F. & Deamer, D. W. Production of RNA by a polymerase protein encapsulated within phospholipid vesicles. J. Mol. Evol. 39, 555–559 (1994)
Monnard, P. A., Luptak, A. & Deamer, D. W. Models of primitive cellular life: polymerases and templates in liposomes. Phil. Trans. R. Soc. Lond. B 362, 1741–1750 (2007)
Apel, C. L., Deamer, D. W. & Mautner, M. N. Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim. Biophys. Acta 1559, 1–9 (2002)
Blochliger, E., Blocher, M., Walde, P. & Luisi, P. L. Matrix effect in the size distribution of fatty acid vesicles. J. Phys. Chem. B 102, 10383–10390 (1998)
Hargreaves, W. R. & Deamer, D. W. Liposomes from ionic, single-chain amphiphiles. Biochemistry 17, 3759–3768 (1978)
Chen, I. A., Salehi-Ashtiani, K. & Szostak, J. W. RNA catalysis in model protocell vesicles. J. Am. Chem. Soc. 127, 13213–13219 (2005)
Chen, I. A., Roberts, R. W. & Szostak, J. W. The emergence of competition between model protocells. Science 305, 1474–1476 (2004)
Chen, I. A. & Szostak, J. W. A kinetic study of the growth of fatty acid vesicles. Biophys. J. 87, 988–998 (2004)
Hanczyc, M. M., Fujikawa, S. M. & Szostak, J. W. Experimental models of primitive cellular compartments: encapsulation, growth, and division. Science 302, 618–622 (2003)
Chen, P. Y., Pearce, D. & Verkman, A. S. Membrane water and solute permeability determined quantitatively by self-quenching of an entrapped fluorophore. Biochemistry 27, 5713–5718 (1988)
Sacerdote, M. G. & Szostak, J. W. Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proc. Natl Acad. Sci. USA 102, 6004–6008 (2005)
Israelachvili, J. N. Intermolecular and Surface Forces (Academic, London, 1992)
Lande, M. B., Donovan, J. M. & Zeidel, M. L. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J. Gen. Physiol. 106, 67–84 (1995)
Rowat, A. C., Keller, D. & Ipsen, J. H. Effects of farnesol on the physical properties of DMPC membranes. Biochim. Biophys. Acta 1713, 29–39 (2005)
Deamer, D. W. Boundary structures are formed by organic components of the Murchison carbonaceous chondrite. Nature 317, 792–794 (1985)
Huang, Y. et al. Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous meteorites. Geochim. Cosmochim. Acta 69, 1073–1084 (2005)
McCollom, T. M., Ritter, G. & Simoneit, B. R. Lipid synthesis under hydrothermal conditions by Fischer–Tropsch-type reactions. Orig. Life Evol. Biosph. 29, 153–156 (1999)
Khalil, M. M. Complexation equilibria and determination of stability constants of binary and ternary complexes with ribonucleotides (AMP, ADP, and ATP) and salicylhydroxamic acid as ligands. J. Chem. Eng. Data 45, 70–74 (2000)
Westheimer, F. H. Why nature chose phosphates. Science 235, 1173–1178 (1987)
Eschenmoser, A. The search for the chemistry of life’s orgin. Tetrahedron 63, 12821–12844 (2007)
Ferris, J. P., Hill, A. R., Liu, R. & Orgel, L. E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381, 59–61 (1996)
Kozlov, I. A., Pitsch, S. & Orgel, L. E. Oligomerization of activated d- and l-guanosine mononucleotides on templates containing d- and l-deoxycytidylate residues. Proc. Natl Acad. Sci. USA 95, 13448–13452 (1998)
Tohidi, M., Zielinski, W. S., Chen, C. H. & Orgel, L. E. Oligomerization of the 3′-amino-3′deoxyguanosine-5′phosphorimidazolidate on a d(CpCpCpCpC) template. J. Mol. Evol. 25, 97–99 (1987)
Wilson, M. A. & Pohorille, A. Mechanism of unassisted ion transport across membrane bilayers. J. Am. Chem. Soc. 118, 6580–6587 (1996)
Chen, I. A. & Szostak, J. W. Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles. Proc. Natl Acad. Sci. USA 101, 7965–7970 (2004)
Paula, S. G., Volkov, A. G., Van Hoek, A. N., Haines, T. H. & Deamer, D. W. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys. J. 70, 339–348 (1996)
Hagenbuch, P., Kervio, E., Hochgesand, A., Plutowski, U. & Clemens, R. Chemical primer extension: efficiently determining single nucleotides in DNA. Angew. Chem. Int. Edn Engl. 44, 6588–6592 (2005)
Chen, I. A., Hanczyc, M. M., Sazani, P. L. & Szostak, J. W. in The RNA World (eds Gesteland, R. F., Cech, T. R. & Atkins, J. F.) 57–88 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2006)
Morowitz, H. J. Beginnings of Cellular Life: Metabolism Recapitulates Biogenesis (Yale Univ. Press, New Haven, 2004)
Wachtershauser, G. Evolution of the first metabolic cycles. Proc. Natl Acad. Sci. USA 87, 200–204 (1990)
Danilov, L. L. & Chojnacki, T. A simple procedure for preparing dolichyl monophosphate by the use of POCl3 . FEBS Lett. 131, 310–312 (1981)
Guernelli, S. et al. Supramolecular complex formation: a study of the interactions between β-cyclodextrin and some different classes of organic compounds by ESI-MS, surface tension measurements, and UV/Vis and 1H NMR spectroscopy. Eur. J. Org. Chem. 24, 4765–4776 (2003)
Nelson, A. K. & Toy, A. D. F. The preparation of long-chain monoalkyl phosphates from pyrophosphoric acid and alcohols. Inorg. Chem. 2, 775–777 (1963)
Kawana, M. & Kuzuhara, H. General method for the synthesis of 2′-azido-2′,3′-dideoxynucleosides by the use of [1,2]-hydride shift and β-elimination reactions. J. Chem. Soc. Perkin Trans. I 4, 469–478 (1992)
Acknowledgements
This work was supported by grants from the NASA Exobiology Program (EXB02-0031-0018) and the NSF (CHE-0434507) to J.W.S. J.W.S. is an Investigator of the Howard Hughes Medical Institute. S.S.M. was supported by the NIH (F32 GM07450601). We thank I. Chen, M. Hanczyc, R. Bruckner, T. Zhu and Q. Dufton for discussions, and J. Iwasa for Fig. 1 and Supplementary Fig. 5.
Author Contributions Permeability experiments were performed by S.S.M. J.P.S. performed primer-extension experiments. M.K. synthesized 2′-aminoguanosine. S.T. and D.A.T. contributed to the development of the encapsulated primer-extension system. All authors helped to design the experiments and discussed the results. S.S.M., J.P.S. and J.W.S. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Figures S1-S7 with Legends and Supplementary Table S1. (PDF 1451 kb)
Rights and permissions
About this article
Cite this article
Mansy, S., Schrum, J., Krishnamurthy, M. et al. Template-directed synthesis of a genetic polymer in a model protocell. Nature 454, 122–125 (2008). https://doi.org/10.1038/nature07018
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07018
This article is cited by
-
Life on Earth can grow on extraterrestrial organic carbon
Scientific Reports (2024)
-
An inorganic mineral-based protocell with prebiotic radiation fitness
Nature Communications (2023)
-
Scientific progress, normative discussions, and the pragmatic account of definitions of life
Synthese (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.