Abstract
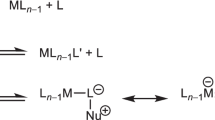
Transition-metal catalysts containing gold present new opportunities for chemical synthesis, and it is therefore not surprising that these complexes are beginning to capture the attention of the chemical community. Cationic phosphine–gold(i) complexes are especially versatile and selective catalysts for a growing number of synthetic transformations. The reactivity of these species can be understood in the context of theoretical studies on gold; relativistic effects are especially helpful in rationalizing the reaction manifolds available to gold catalysts. This Review draws on experimental and computational data to present our current understanding of homogeneous gold catalysis, focusing on previously unexplored reactivity and its application to the development of new methodology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Pyykkö, P. Theoretical chemistry of gold. II. Inorg. Chim. Acta 358, 4113–4130 (2005)
Pyykkö, P. Theoretical chemistry of gold. Angew. Chem. Int. Ed. 43, 4412–4456 (2004)
McKelvey, D. R. Relativistic effects on chemical properties. J. Chem. Educ. 60, 112–116 (1983)
Pyykkö, P. & Desclaux, J. P. Relativity and the periodic system of elements. Acc. Chem. Res. 12, 276–281 (1979)
Desclaux, J. P. & Pyykkö, P. Dirac–Fock one-center calculations—molecules CuH, AgH and AuH including P-type symmetry functions. Chem. Phys. Lett. 39, 300–303 (1976)
Scherbaum, F., Grohmann, A., Huber, B., Krüger, C. & Schmidbaur, H. ‘Aurophilicity’ as a consequence of relativistic effects: The hexakis(triphenylphosphaneaurio)methane dication [(Ph3P)Au]6C2+. Angew. Chem. Int. Edn Engl. 27, 1544–1546 (1988)
Carvajal, M. A., Novoa, J. J. & Alvarez, S. Choice of coordination number in d10 complexes of Group 11 metals. J. Am. Chem. Soc. 126, 1465–1477 (2004)
Schwerdtfeger, P., Hermann, H. L. & Schmidbaur, H. Stability of the gold(I)–phosphine bond. A comparison with other Group 11 elements. Inorg. Chem. 42, 1334–1342 (2003)
Nakanishi, W., Yamanaka, M. & Nakamura, E. Reactivity and stability of organocopper(I), silver(I), and gold(I) ate compounds and their trivalent derivatives. J. Am. Chem. Soc. 127, 1446–1453 (2005)
Komiya, S. & Kochi, J. K. Electrophilic cleavage of organogold complexes with acids—mechanism of reductive elimination of dialkyl(aniono)gold(III) species. J. Am. Chem. Soc. 98, 7599–7607 (1976)
Komiya, S., Albright, T. A., Hoffmann, R. & Kochi, J. K. Reductive elimination and isomerization of organogold complexes—theoretical studies of trialkylgold species as reactive intermediates. J. Am. Chem. Soc. 98, 7255–7265 (1976)
Tamaki, A. & Kochi, J. K. Oxidative addition in coupling of alkylgold(I) with alkyl-halides. J. Organometall. Chem. 64, 411–425 (1974)
Hashmi, A. S. K. Homogeneous catalysis by gold. Gold Bull. 37, 51–65 (2004)
Hashmi, A. S. K. Homogeneous gold catalysts and alkynes: A successful liaison. Gold Bull. 36, 3–9 (2003)
Fukuda, Y., Utimoto, K. & Nozaki, H. Preparation of 2,3,4,5-tetrahydropyridines from 5-alkynylamines under the catalytic action of Au(III). Heterocycles 25, 297–300 (1987)
Asao, N., Takahashi, K., Lee, S., Kasahara, T. & Yamamoto, Y. AuCl3-catalyzed benzannulation: Synthesis of naphthyl ketone derivatives from o-alkynylbenzaldehydes with alkynes. J. Am. Chem. Soc. 124, 12650–12651 (2002)
Hashmi, A. S. K., Frost, T. M. & Bats, J. W. Highly selective gold-catalyzed arene synthesis. J. Am. Chem. Soc. 122, 11553–11554 (2000)
Hashmi, A. S. K., Schwarz, L., Choi, J.-H. & Frost, T. M. A new gold-catalyzed C–C bond formation. Angew. Chem. Int. Ed. 39, 2285–2288 (2000)
Fukuda, Y. & Utimoto, K. Effective transformation of unactivated alkynes into ketones or acetals by means of Au(III) catalyst. J. Org. Chem. 56, 3729–3731 (1991)
Yao, X. & Li, C. J. Water-triggered and gold(I)-catalyzed cascade addition/cyclization of terminal alkynes with ortho-alkynylaryl aldehyde. Org. Lett. 8, 1953–1955 (2006)
Arcadi, A., Bianchi, G., Di Giuseppe, S. & Marinelli, F. Gold catalysis in the reactions of 1,3-dicarbonyls with nucleophiles. Green Chem. 5, 64–67 (2003)
Teles, J. H., Brode, S. & Chabanas, M. Cationic gold(I) complexes: Highly efficient catalysts for the addition of alcohols to alkynes. Angew. Chem. Int. Ed. 37, 1415–1418 (1998)
Kennedy-Smith, J. J., Staben, S. T. & Toste, F. D. Gold(I)-catalyzed Conia-ene reaction of beta-ketoesters with alkynes. J. Am. Chem. Soc. 126, 4526–4527 (2004)
Staben, S. T., Kennedy-Smith, J. J. & Toste, F. D. Gold-catalyzed 5-endo-dig carbocyclization of acetylenic dicarbonyl compounds. Angew. Chem. Int. Ed. 43, 5350–5352 (2004)
Reetz, M. T. & Sommer, K. Gold-catalyzed hydroarylation of alkynes. Eur. J. Org. Chem. 2003, 3485–3496 (2003)
Nevado, C. & Echavarren, A. M. Intramolecular hydroarylation of alkynes catalyzed by platinum or gold: Mechanism and endo selectivity. Chem. Eur. J. 11, 3155–3164 (2005)
Ferrer, C. & Echavarren, A. M. Gold-catalyzed intramolecular reaction of indoles with alkynes: Facile formation of eight-membered rings and an unexpected allenylation. Angew. Chem. Int. Ed. 45, 1105–1109 (2006)
Antoniotti, S., Genin, E., Michelet, W. & Genet, J. P. Highly efficient access to strained bicyclic ketals via gold-catalyzed cycloisomerization of bis-homopropargylic diols. J. Am. Chem. Soc. 127, 9976–9977 (2005)
Buzas, A. & Gagosz, F. Gold(I)-catalyzed formation of 4-alkylidene-1,3-dioxolan-2-ones from propargylic tert-butyl carbonates. Org. Lett. 8, 515–518 (2006)
Mizushima, E., Hayashi, T. & Tanaka, M. Au(I)-catalyzed highly efficient intermolecular hydroamination of alkynes. Org. Lett. 5, 3349–3352 (2003)
Zhang, L. M. Tandem Au-catalyzed 3,3-rearrangement-[2 + 2] cycloadditions of propargylic esters: Expeditious access to highly functionalized 2,3-indoline-fused cyclobutanes. J. Am. Chem. Soc. 127, 16804–16805 (2005)
Suhre, M. H., Reif, M. & Kirsch, S. F. Gold(I)-catalyzed synthesis of highly substituted furans. Org. Lett. 7, 3925–3927 (2005)
Morita, N. & Krause, N. The first gold-catalyzed C–S bond formation: Cycloisomerization of alpha-thioallenes to 2,5-dihydrothiophenes. Angew. Chem. Int. Ed. 45, 1897–1899 (2006)
Zhang, J. L., Yang, C. G. & He, C. Gold(I)-catalyzed intra- and intermolecular hydroamination of unactivated olefins. J. Am. Chem. Soc. 128, 1798–1799 (2006)
Yao, X. & Li, C. J. Highly efficient addition of activated methylene compounds to alkenes catalyzed by gold and silver. J. Am. Chem. Soc. 126, 6884–6885 (2004)
Brouwer, C. & He, C. Efficient gold-catalyzed hydroamination of 1,3-dienes. Angew. Chem. Int. Ed. 45, 1744–1747 (2006)
Nguyen, R. V., Yao, X. & Li, C. J. Highly efficient gold-catalyzed atom-economical annulation of phenols with dienes. Org. Lett. 8, 2397–2399 (2006)
Rosenfeld, D. C., Shekhar, S., Takemiya, A., Utsunomiya, M. & Hartwig, J. F. Hydroamination and hydroalkoxylation catalyzed by triflic acid. Parallels to reactions initiated with metal triflates. Org. Lett. 8, 4179–4182 (2006)
Li, Z. et al. Bronsted acid catalyzed addition of phenols, carboxylic acids, and tosylamides to simple olefins. Org. Lett. 8, 4175–4178 (2006)
Schwerdtfeger, P., Boyd, P. D. W., Burrell, A. K., Robinson, W. T. & Taylor, M. J. Relativistic effects in gold chemistry. 3. Gold(I) complexes. Inorg. Chem. 29, 3593–3607 (1990)
Hertwig, R. H. et al. A comparative computational study of cationic coinage metal-ethylene complexes (C2H4)M+ (M = Cu, Ag, and Au). J. Phys. Chem. 100, 12253–12260 (1996)
Nechaev, M. S., Rayon, V. M. & Frenking, G. Energy partitioning analysis of the bonding in ethylene and acetylene complexes of Group 6, 8, and 11 metals: (CO)(5)TM-C2Hx and Cl4TM-C2Hx (TM = Cr, Mo, W), (CO)(4)TM-C2Hx (TM = Fe, Ru, Os), and TM+-C2Hx (TM = Cu, Ag, Au). J. Phys. Chem. A 108, 3134–3142 (2004)
Fleming, I. Frontier Orbitals and Organic Chemical Reactions (Wiley, Chichester, 1976)
Cinellu, M. A. et al. Reactions of gold(III) oxo complexes with cyclic alkenes. Angew. Chem. Int. Ed. 44, 6892–6895 (2005)
Hashmi, A. S. K., Weyrauch, J. P., Frey, W. & Bats, J. W. Gold catalysis: Mild conditions for the synthesis of oxazoles from N-propargylcarboxamides and mechanistic aspects. Org. Lett. 6, 4391–4394 (2004)
Nevado, C. & Echavarren, A. M. Transition metal-catalyzed hydroarylation of alkynes. Synthesis 167–182 (2005)
Sromek, A. W., Rubina, M. & Gevorgyan, V. 1,2-Halogen migration in haloallenyl ketones: Regiodivergent synthesis of halofurans. J. Am. Chem. Soc. 127, 10500–10501 (2005)
Straub, B. F. Gold(I) or gold(III) as active species in AuCl3-catalyzed cyclization/cycloaddition reactions? A DFT study. Chem. Commun. 1726–1728 (2004)
Markham, J. P., Staben, S. T. & Toste, F. D. Gold(I)-catalyzed ring expansion of cyclopropanols and cyclobutanols. J. Am. Chem. Soc. 127, 9708–9709 (2005)
Zhang, Z. et al. Highly active Au(I) catalyst for the intramolecular exo-hydrofunctionalization of allenes with carbon, nitrogen, and oxygen nucleophiles. J. Am. Chem. Soc. 128, 9066–9073 (2006)
Marion, N., Díez-González, S., de Frémont, P., Noble, A. R. & Nolan, S. P. AuI-catalyzed tandem [3,3] rearrangement–intramolecular hydroarylation: Mild and efficient formation of substituted indenes. Angew. Chem. Int. Edn 45, 3647–3650 (2006)
Sherry, B. D. & Toste, F. D. Gold(I)-catalyzed propargyl Claisen rearrangement. J. Am. Chem. Soc. 126, 15978–15979 (2004)
Hashmi, A. S. K., Weyrauch, J. P., Rudolph, M. & Kurpejovic, E. Gold catalysis: the benefits of N and N,O ligands. Angew. Chem. Int. Ed. 43, 6545–6547 (2004)
Zhou, C. Y., Chan, P. W. H. & Che, C. M. Gold(III) porphyrin-catalyzed cycloisomerization of allenones. Org. Lett. 8, 325–328 (2006)
Irikura, K. K. & Goddard, W. A. Energetics of third-row transition metal methylidene ions MCH2+ (M = La, Hf, Ta, W, Re, Os, Ir, Pt, Au). J. Am. Chem. Soc. 116, 8733–8740 (1994)
Heinemann, C., Hertwig, R. H., Wesendrup, R., Koch, W. & Schwarz, H. Relativistic effects on bonding in cationic transition-metal–carbene complexes—a density-functional study. J. Am. Chem. Soc. 117, 495–500 (1995)
Barysz, M. & Pyykkö, P. Strong chemical bonds to gold. High level correlated relativistic results for diatomic AuBe+, AuC+, AgMg+, and AuSi+. Chem. Phys. Lett. 285, 398–403 (1998)
Aguirre, F., Husband, J., Thompson, C. J. & Metz, R. B. Gas-phase photodissociation of AuCH2+: the dissociation threshold of jet-cooled and rotationally thermalized ions. Chem. Phys. Lett. 318, 466–470 (2000)
Xu, Q., Imamura, Y., Fujiwara, M. & Souma, Y. A new gold catalyst: Formation of gold(I) carbonyl, [Au(CO)n]+ (n = 1, 2), in sulfuric acid and its application to carbonylation of olefins. J. Org. Chem. 62, 1594–1598 (1997)
Chatt, J. & Duncanson, L. A. Olefin co-ordination compounds. 3. Infra-red spectra and structure—attempted preparation of acetylene complexes. J. Chem. Soc. 2939–2947 (1953)
Dewar, J. S. A review of the pi-complex theory. Bull. Soc. Chim. Fr. 18, C71–C79 (1951)
deFremont, P., Scott, N. M., Stevens, E. D. & Nolan, S. P. Synthesis and structural characterization of N-heterocyclic carbene gold(I) complexes. Organometallics 24, 2411–2418 (2005)
Raubenheimer, H. G., Esterhuysen, M. W., Timoshkin, A., Chen, Y. & Frenking, G. Electrophilic addition of Ph3PAu+ to anionic alkoxy Fischer-type carbene complexes: A novel approach to metal-stabilized bimetallic vinyl ether complexes. Organometallics 21, 3173–3181 (2002)
Nakamura, I., Sato, T. & Yamamoto, Y. Gold-catalyzed intramolecular carbothiolation of alkynes: Synthesis of 2,3-disubstituted benzothiophenes from (alpha-alkoxy alkyl) (ortho-alkynyl phenyl) sulfides. Angew. Chem. Int. Ed. 45, 4473–4475 (2006)
Dube, P. & Toste, F. D. Synthesis of indenyl ethers by gold(I)-catalyzed intramolecular carboalkoxylation of alkynes. J. Am. Chem. Soc. 128, 12062–12063 (2006)
Nieto-Oberhuber, C. et al. Gold(I)-catalyzed cyclizations of 1,6-enynes: Alkoxycyclizations and exo/endo skeletal rearrangements. Chem. Eur. J. 12, 1677–1693 (2006)
Nieto-Oberhuber, C. et al. Cationic gold(I) complexes: Highly alkynophilic catalysts for the exo- and endo-cyclization of enynes. Angew. Chem. Int. Ed. 43, 2402–2406 (2004)
Mamane, V., Gress, T., Krause, H. & Fürstner, A. Platinum- and gold-catalyzed cycloisomerization reactions of hydroxylated enynes. J. Am. Chem. Soc. 126, 8654–8655 (2004)
Luzung, M. R., Markham, J. P. & Toste, F. D. Catalaytic isomerization of 1,5-enynes to bicyclo[3.1.0]hexenes. J. Am. Chem. Soc. 126, 10858–10859 (2004)
Zhang, L. & Kozmin, S. A. Gold-catalyzed assembly of heterobicyclic systems. J. Am. Chem. Soc. 127, 6962–6963 (2005)
Fürstner, A., Stelzer, F. & Szillat, H. Platinum-catalyzed cycloisomerization reactions of enynes. J. Am. Chem. Soc. 123, 11863–11869 (2001)
Mendez, M., Mamane, V. & Fürstner, A. Platinum-catalyzed skeletal rearrangement reactions: Generating structural diversity by a uniform mechanism. ChemTracts Org. Chem. 16, 397–425 (2003)
Fürstner, A. & Mamane, V. Flexible synthesis of phenanthrenes by a PtCl2-catalyzed cycloisomerization reaction. J. Org. Chem. 67, 6264–6267 (2002)
Chatani, N., Inoue, H., Kotsuma, T. & Murai, S. Skeletal reorganization of enynes to 1-vinylcycloalkenes catalyzed by GaCl3 . J. Am. Chem. Soc. 124, 10294–10295 (2002)
Mamane, V., Hannen, P. & Fürstner, A. Synthesis of phenanthrenes and polycyclic heteroarenes by transition-metal catalyzed cycloisomerization reactions. Chem. Eur. J. 10, 4556–4575 (2004)
Christian, B. Electrophilic activation and cycloisomerization of enynes: A new route to functional cyclopropanes. Angew. Chem. Int. Ed. 44, 2328–2334 (2005)
Ma, S., Yu, S. & Gu, Z. Gold-catalyzed cyclization of enynes. Angew. Chem. Int. Ed. 45, 200–203 (2006)
Furstner, A., Davies, P. W. & Gress, T. Cyclobutenes by platinum-catalyzed cycloisomerization reactions of enynes. J. Am. Chem. Soc. 127, 8244–8245 (2005)
Oi, S., Tsukamoto, I., Miyano, S. & Inoue, Y. Cationic platinum-complex-catalyzed skeletal reorganization of enynes. Organometallics 20, 3704–3709 (2001)
BhanuPrasad, B. A., Yoshimoto, F. K. & Sarpong, R. Pt-catalyzed pentannulations from in situ generated metallo-carbenoids utilizing propargylic esters. J. Am. Chem. Soc. 127, 12468–12469 (2005)
Ito, Y., Sawamura, M. & Hayashi, T. Catalytic asymmetric aldol reaction—reaction of aldehydes with isocyanoacetate catalyzed by a chiral ferrocenylphosphine-gold(I) complex. J. Am. Chem. Soc. 108, 6405–6406 (1986)
Munoz, M. P., Adrio, J., Carretero, J. C. & Echavarren, A. M. Ligand effects in gold- and platinum-catalyzed cyclization of enynes: Chiral gold complexes for enantioselective alkoxycyclization. Organometallics 24, 1293–1300 (2005)
Shi, X., Gorin, D. J. & Toste, F. D. Synthesis of 2-cyclopentenones by gold(I)-catalyzed Rautenstrauch rearrangement. J. Am. Chem. Soc. 127, 5802–5803 (2005)
Rautenstrauch, V. 2-Cyclopentenones from 1-ethynyl-2-propenyl acetates. J. Org. Chem. 49, 950–952 (1984)
Faza, O. N., Lopez, C. S., Alvarez, R. & de Lera, A. R. Mechanism of the gold(I)-catalyzed Rautenstrauch rearrangement: A center-to-helix-to-center chirality transfer. J. Am. Chem. Soc. 128, 2434–2437 (2006)
Fehr, C. & Galindo, J. Synthesis of (-)-cubebol by face-selective platinum-, gold-, or copper-catalyzed cycloisomerization: Evidence for chirality transfer. Angew. Chem. Int. Edn 45, 2901–2904 (2006)
Furstner, A. & Hannen, P. Carene terpenoids by gold-catalyzed cycloisomerization reactions. Chem. Commun. 2546–2547 (2004)
Furstner, A. & Hannen, P. Platinum- and gold-catalyzed rearrangement reactions of propargyl acetates: Total syntheses of (-)-alpha-cubebene, (-)-cubebol, sesquicarene and related terpenes. Chem. Eur. J. 12, 3006–3019 (2006)
Johansson, M. J., Gorin, D. J., Staben, S. T. & Toste, F. D. Gold(I)-catalyzed stereoselective olefin cyclopropanation. J. Am. Chem. Soc. 127, 18002–18003 (2005)
Miki, K., Ohe, K. & Uemura, S. A new ruthenium-catalyzed cyclopropanation of alkenes using propargylic acetates as a precursor of vinylcarbenoids. Tetrahedr. Lett. 44, 2019–2022 (2003)
Gorin, D. J., Davis, N. R. & Toste, F. D. Gold(I)-catalyzed intramolecular acetylenic Schmidt reaction. J. Am. Chem. Soc. 127, 11260–11261 (2005)
Hashmi, A. S. K., Blanco, M. C., Kurpejovic, E., Frey, W. & Bats, J. W. Gold catalysis: First applications of cationic binuclear gold(I) complexes and the first intermolecular reaction of an alkyne with a furan. Adv. Synth. Catal. 348, 709–713 (2006)
Fructos, M. R. et al. A gold catalyst for carbene-transfer reactions from ethyl diazoacetate. Angew. Chem. Int. Ed. 44, 5284–5288 (2005)
Hoffmann-Roder, A. & Krause, N. The golden gate to catalysis. Org. Biomol. Chem. 3, 387–391 (2005)
Hashmi, A. S. K. The catalysis gold rush: New claims. Angew. Chem. Int. Ed. 44, 6990–6993 (2005)
Pitzer, K. S. Relativistic effects on chemical properties. Acc. Chem. Res. 12, 272–276 (1979)
Pyykkö, P. Relativistic effects in structural chemistry. Chem. Rev. 88, 563–594 (1988)
Norrby, L. J. Why is mercury liquid—or, why do relativistic effects not get into chemistry textbooks?. J. Chem. Educ. 68, 110–113 (1991)
Bagus, P. S., Lee, Y. S. & Pitzer, K. S. Effects of relativity and of lanthanide contraction on atoms from hafnium to bismuth. Chem. Phys. Lett. 33, 408–411 (1975)
Desclaux, J. P. Relativistic Dirac–Fock expectation values for atoms with Z = 1 to Z = 120. Atom. Data Nucl. Data Tables 12, 311–406 (1973)
Acknowledgements
We thank P. Pyykkö for discussions during the preparation of this manuscript. Funding from the University of California, Berkeley, NIHGMS, Merck Research Laboratories, Bristol-Myers Squibb, Amgen Inc., DuPont, GlaxoSmithKline, Eli Lilly & Co., Pfizer, AstraZeneca, Abbott, Boehringer Ingelheim, Novartis and Roche is gratefully acknowledged. D.J.G. is an ACS Organic Division predoctoral fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gorin, D., Toste, F. Relativistic effects in homogeneous gold catalysis. Nature 446, 395–403 (2007). https://doi.org/10.1038/nature05592
Issue Date:
DOI: https://doi.org/10.1038/nature05592
This article is cited by
-
Intramolecular hydroamination catalysed by gold nanoparticles deposited on fibrillated cellulose
Scientific Reports (2022)
-
Gold carbene complexes and beyond: new avenues in gold(I)-carbon coordination chemistry
Gold Bulletin (2022)
-
Catalytic Hydration of Aromatic Alkynes to Ketones over H-MFI Zeolites
Chemical Research in Chinese Universities (2022)
-
Synthesis of N-heterocyclic carbene gold(I) complexes
Nature Protocols (2021)
-
Gold-catalyzed formation of substituted aminobenzophenone derivatives via intramolecular 6-endo-dig cyclization
Journal of Chemical Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.