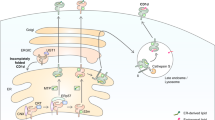
Abstract
CD1, a conserved family of major histocompatibility (MHC)-like glycoproteins in mammals, specializes in capturing lipid rather than peptide antigen for presentation to T lymphocytes. The principles and mechanisms of this newly discovered immune strategy differ markedly from those governing classical MHC–peptide presentation. They might be exploited for the design of new lipid-based microbial vaccines and adjuvants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Calabi, F., Jarvis, J. M., Martin, L. H. & Milstein, C. Two classes of CD1 genes. Eur. J. Immunol. 19, 285–292 (1989).
Porcelli, S. A. & Modlin, R. L. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids . Annu. Rev. Immunol. 17, 297– 329 (1999).
Beckman, E. M. et al. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372, 691– 694 (1994).
Moody, D. B. et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 278, 283–286 (1997).
Sieling, P. A. et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269, 227– 230 (1995).
Moody, D. B. et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404, 884–888 (2000).
Lee, R. E., Brennan, P. J. & Besra, G. S. Mycobacterium tuberculosis cell envelope. Curr. Top. Microbiol. Immunol. 215, 1– 27 (1996).
Natori, T., Morita, M., Akimoto, K. & Koezuka, Y. Agelasphins, novel antitumor and immunostimulatory cerebrosides from the sponge Agelas mauritianus . Tetrahedron 50, 2771– 2784 (1994).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Shamshiev, A. et al. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29, 1667–1675 ( 1999).
Castano, A. R. et al. Peptide binding and presentation by mouse CD1. Science 269, 223–226 ( 1995).
Zeng, Z. H. et al. The crystal structure of murine CD1: an MHC-like fold with a large hydrophobic antigen binding groove. Science 277, 339–345 (1997).
Porcelli, S. A. & Brenner, M. B. Antigen presentation: mixing oil and water. Curr. Biol. 7, R508 –R511 (1997).
Naidenko, O. V. et al. Binding and antigen presentation of ceramide-containing glycolipids by soluble mouse and human CD1d molecules. J. Exp. Med. 190, 1069–1080 (1999).
Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. In vivo identification of glycolipid antigen specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191, 1895–1903 ( 2000).
Joyce, S. et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol . Science 279, 1541–1544 (1998).
Ernst, W. A. et al. Molecular interaction of CD1b with lipoglycan antigens. Immunity 8, 331–340 ( 1998).
Burdin, N. et al. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα 14+ NK T lymphocytes. J. Immunol. 161, 3271–3281 ( 1998).
Sugita, M. et al. Separate pathways for antigen presentation by CD1 molecules . Immunity 11, 743–752 (1999).
Schaible, U. E., Hagens, K., Fischer, K., Collins, H. L. & Kaufmann, S. H. Intersection of group I CD1 molecules and mycobacteria in different intracellular compartments of dendritic cells. J. Immunol. 164, 4843–4852 (2000).
Jackman, R. M. et al. The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity 8, 341–351 ( 1998).
Chiu, Y. H. et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 189, 103–110 (1999).
Briken, V., Jackman, R. M., Watts, G. F. M., Rogers, R. A. & Porcelli, S. A. Human CD1b and CD1c isoforms survey different intracellular compartments for the presentation of microbial lipid antigens. J. Exp. Med. 192, 281– 288 (2000)
Sieling, P. A. et al. Evidence for human CD4− T cells in the CD1-restricted repertoire: derivation of mycobacteria-reactive T cells from leprosy lesions. J. Immunol. 164, 4790– 4796 (2000).
Bendelac, A., Rivera, M. N., Park, S.-H & Roark, J. H. Mouse CD1-specific NK1 T cells. Development, specificity, and function. Annu. Rev. Immunol. 15, 535–562 (1997).
Stenger, S. et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276, 1684– 1687 (1997).
Tatu, C., Ye, J., Arnold, L. W. & Clarke, S. H. Selection at multiple checkpoints focuses V(H)12 B cell differentiation toward a single B-1 cell specificity. J. Exp. Med. 190, 903–914 (1999).
Benedict, C. L. & Kearney, J. F. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity 10, 607 –617 (1999).
Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P. & Salazar-Mather, T. P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 (1999).
Bendelac, A. et al. CD1 recognition by mouse NK1+ T lymphocytes . Science 268, 863–865 (1995).
Spada, F. M. et al. Self-recognition of CD1 by γ/δ T cells: implications for innate immunity. J. Exp. Med. 191, 937 –948 (2000).
Park, S. H., Benlagha, K., Lee, D., Balish, E. & Bendelac, A. Unaltered phenotype, tissue distribution and function of Vα14(+) NKT cells in germ-free mice. Eur. J. Immunol. 30, 620–625 ( 2000).
Schofield, L. et al. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283, 225–229 (1999).
Molano, A. et al. Cutting edge. The IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of glycosylphosphatidylinositols in NKT cell activation and anti-malarial responses. J. Immunol. 164, 5005–5009 ( 2000).
Gumperz, J. E. et al. Murine CD1d-restricted T cell recognition of cellular lipids . Immunity 12, 211–221 (2000).
Burdin, N., Brossay, L. & Kronenberg, M. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29, 2014–2025 ( 1999).
Carnaud, C. et al. Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163, 4647–4650 (1999).
Kitamura, H. et al. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189, 1121–1128 (1999).
Hammond, K. J. L. et al. α/β-T cell receptor (TCR) CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J. Exp. Med. 187, 1047– 1056 (1998).
Lehuen, A. et al. Overexpression of natural killer T cells protects Vα14- Jα281 transgenic nonobese diabetic mice against diabetes. J. Exp. Med. 188, 1831–1839 (1998).
Wilson, S. B. et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type I diabetes. Nature 391, 177– 181 (1998).
Smyth, M. J. et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191, 661– 668 (2000).
Cui, J. et al. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J. Exp. Med. 190, 783–792 (1999).
Singh, N. et al. Cutting edge: activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163, 2373–2377 ( 1999).
Shinkai, K. & Locksley, R. M. CD1, tuberculosis, and the evolution of major histocompatibility complex molecules. J. Exp. Med. 191, 907–914 (2000).
Beldjord, K., Beldjord, C., Macintyre, E., Even, P. & Sigaux, F. Peripheral selection of V δ 1+ cells with restricted T cell receptor delta gene junctional repertoire in the peripheral blood of healthy donors. J. Exp. Med. 178, 121–127 (1993).
Besra, G. & Chatterjee, D. in Tuberculosis: Pathogenesis, Protection and Control. (ed. Bloom, B.) 285–306 (American Society for Microbiology, Washington, DC, 1994).
Almeida, I. C. et al. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 19, 1476–1485 (2000).
Acknowledgements
We thank S. Balk, M. Bonneville, M. Ferguson, J.-J. Fournie, S. Joyce, S. Kaufmann, Y. Koezuka, H.R. MacDonald, I. Orme, S. Porcelli, L. Schofield, L. Teyton, C.-R. Wang and numerous other colleagues for helpful discussions and for sharing unpublished results; P. Brennan for permission to reproduce his model of the mycobacterial cell wall; members of our laboratory for stimulating discussions; H. Piper for help in the preparation of Fig. 2 ; and B. Jabri and P. Matzinger for critical review of the manuscript. This work was supported by grants from the NIH, American Cancer Society and Juvenile Diabetes Foundation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Park, SH., Bendelac, A. CD1-restricted T-cell responses and microbial infection. Nature 406, 788–792 (2000). https://doi.org/10.1038/35021233
Published:
Issue Date:
DOI: https://doi.org/10.1038/35021233
This article is cited by
-
Targeted disruption of CD1d prevents NKT cell development in pigs
Mammalian Genome (2015)
-
PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions
Mucosal Immunology (2010)
-
Innate and adaptive immunity in atherosclerosis
Seminars in Immunopathology (2009)
-
Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections
Nature (2005)
-
Chicken CD1 genes are located in the MHC: CD1 and endothelial protein C receptor genes constitute a distinct subfamily of class-I-like genes that predates the emergence of mammals
Immunogenetics (2005)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.