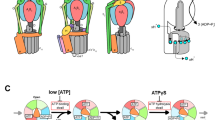
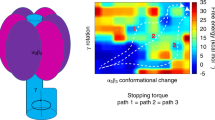
Abstract
ATP synthase is the universal enzyme that manufactures ATP from ADP and phosphate by using the energy derived from a transmembrane protonmotive gradient. It can also reverse itself and hydrolyse ATP to pump protons against an electrochemical gradient. ATP synthase carries out both its synthetic and hydrolytic cycles by a rotary mechanism1,2,3,4. This has been confirmed in the direction of hydrolysis5,6 after isolation of the soluble F1 portion of the protein and visualization of the actual rotation of the central ‘shaft’ of the enzyme with respect to the rest of the molecule, making ATP synthase the world's smallest rotary engine. Here we present a model for this engine that accounts for its mechanochemical behaviour in both the hydrolysing and synthesizing directions. We conclude that the F1 motor achieves its high mechanical torque and almost 100% efficiency because it converts the free energy of ATP binding into elastic strain, which is then released by a coordinated kinetic and tightly coupled conformational mechanism to create a rotary torque.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Abrahams, J., Leslie, A., Lutter, R. & Walker, J. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628 (1994).
Boyer, P. The binding change mechanism for ATP synthase—some probabilities and possibilities. Biochim. Biophys. Acta 1140, 215–250 (1993).
Fillingame, R. H. Coupling H+ transport and ATP synthesis in F1Fo-ATP synthases: glimpses of interacting parts in a dynamic molecular machine. J. Exp. Biol. 200, 217–224 (1997).
Cross, R. & Duncan, T. Subunit rotation in FoF1-ATP synthases as a means of coupling proton transport through Foto the binding changes in F1. J. Bioen. Biomembr. 28, 403–408 (1996).
Noji, H., Yasuda, R., Yoshida, M. & Kinosita, K. Direct observation of the rotation of F1-ATPase. Nature 386, 299–302 (1997).
Yasuda, R., Noji, H., Kinosita, K., Motojima, F. & Yoshida, M. Rotation of the γ subunit in F1-ATPase: Evidence that ATP synthase is a rotary motor enzyme. J. Bioen. Biomembr. 29, 207–209 (1997).
Elston, T., Wang, H. & Oster, G. Energy transduction in ATP synthase. Nature 391, 510–514 (1998).
Shirakihara, Y. et al. The crystal structure of the nucleotide-free α3 β3 subcomplex of F1-ATPase from the thermophilic Bacillus PS3 is a symmetric trimer. Structure 5, 825–836 (1997).
Gerstein, M., Lesk, A. & Chothia, C. Structural mechanisms for domain movements in proteins. Biochemistry 33, 6739–6749 (1994).
Gerstein, M. in Protein Motions (ed. Subbiah, S.) 81–90 (Chapman and Hall, New York, 1996).
Alberts, B. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell 92, 291–294 (1998).
Rojnuckarin, A., Kim, S. & Subramaniam, S. Brownian dynamics simulations of protein folding: access to milliseconds time scale and beyond. Proc. Natl Acad. Sci. USA 95, 4288–4292 (1998).
Senior, A. Catalytic sites of Escherichia coli F1-ATPase. J. Bioen. Biomembr. 24, 479–483 (1992).
Al-Shawi, M. & Nakamoto, R. Mechanism of energy coupling in the FoF1-ATP synthase: the uncoupling mutation, γM23K, disrupts the use of binding energy to drive catalysis. Biochemistry 36, 12954–12960 (1997).
Hasler, K., Engelbrecht, S. & Junge, W. Three-stepped rotation of subunits γ and ε in single molecules of F-ATPase as revealed by polarized, confocal fluorometry. FEBS Lett. 426, 301–304 (1998).
Yasuda, R., Noji, H., Kinosita, K. & Yoshida, M. F1ATPase is a stepper motor. Biophys. J. 74, A1 (1998).
Matsuno-Yagi, A. & Hatefi, Y. Kinetic modalities of ATP synthesis: regulation by the mitochondrial respiratory chain. J. Biol. Chem. 261, 14031–14038 (1986).
Groth, G. & Junge, W. Proton slip of the chloroplast ATPase: its nucleotide dependence, energetic threshold, and relation to an alternating site mechanism of catalysis. Biochemistry 32, 8103–8111 (1993).
Yasuda, R., Noji, H., Kinosita, K. & Yoshida, M. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120° steps. Cell 93, 1117–1124 (1998).
Acknowledgements
We thank J. Walker for discussions on the motions of the β-subunit and for videos of the three configurations which simulated our interpolated videos; R. Nakamoto for advice on the switch 1 and 2 interactions; M. Yoshida, K. Kinosita and their co-workers for inspiring the construction of the F1 model by their ingenious experiments; and M. Grabe and K. Kinosita for insightful comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Oster, G. Energy transduction in the F1 motor of ATP synthase. Nature 396, 279–282 (1998). https://doi.org/10.1038/24409
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/24409
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.