Abstract
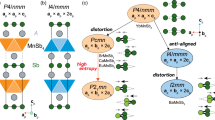
THE polymorphism of NaAlO2 and NaFeO2 is well known. Both oxides form β and γ polymorphs, and although their crystal structures are known, the relationship between the β and γ polymorphs has not been described. This relationship is described here and from it a simple topotactic mechanism for the β⇌γ transformation is deduced. Movement of only half of the cations is necessary to complete the transformation in either direction and this movement is restricted to a single, filled-empty tetrahedron jump, for each cation. The polymorphisms may be summarised: 
 An α form may also be prepared at high pressure5. Both the β and γ polymorphs have ‘tetrahedral structures’, that is, the cations and oxygens are all tetrahedrally coordinated. (The α form has an ordered rock-salt structure.)
An α form may also be prepared at high pressure5. Both the β and γ polymorphs have ‘tetrahedral structures’, that is, the cations and oxygens are all tetrahedrally coordinated. (The α form has an ordered rock-salt structure.)
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bertaut, F., and Blum, P., C. r. hebd. Séanc. Acad. Sci., Paris, 239, 429 (1954).
Bertaut, F., Delapalme, A., and Bassi, G., C. r. hebd. Séanc. Acad. Sci., Paris 257, 421 (1963).
Collongues, R., and Thery, J., Bull. Soc. chim. Fr., 1141 (1959).
Thery, J., Lejus, A-M., Briançon, D., and Collongues, R., Bull, Soc. chim. Fr., 973 (1961).
Reid, A. F., and Ringwood, A. E., Inorg. Chem., 7, 443 (1965).
Ho, S. M., and Douglas, B. E., J. Chem. Educ., 46, 207 (1969).
Marezio, M., Acta crystallogr., 19, 396 (1965).
Bertaut, E. F., Delapalme, A., Bassi, G., Durif-Varambon, A., and Joubert, J. C., Bull. Soc. Fr. Minér. Cristallagr., 88, 103 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WEST, A. NaAlO2 and NaFeO2 Polymorphism. Nature 249, 245–246 (1974). https://doi.org/10.1038/249245a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/249245a0
This article is cited by
-
Crystal structure of NaFeO2 and NaAlO2 and their correlation with ionic conductivity
Ionics (2020)
-
Critical Evaluation and Thermodynamic Optimization of the Na2O-FeO-Fe2O3 System
Metallurgical and Materials Transactions B (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.