Abstract
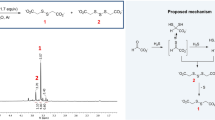
ALTHOUGH a paper by Wieland and Franke in 19281 described the induced oxidation of several hydroxy-acids in the presence of oxidizing ferrous iron, citric acid was not specifically mentioned, and its reaction under these conditions appears not to be well known. We have observed that mixtures of sodium citrate and ferrous sulphate, shaken in air at 28° C. and pH 6.0, absorbed oxygen in excess of that required to oxidize the iron. At the same time carbon dioxide was evolved, and the quantities of carbon dioxide evolved and of excess oxygen absorbed were approximately equimolar. The amounts of excess oxygen (presumably consumed in the oxidation of the citrate), and of carbon dioxide, were proportional to the initial amount of ferrous iron. No reaction was observed in the absence of oxygen. The identification of the decomposition products of citrate, apart from carbon dioxide, was not attempted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wieland, H., and Franke, W., Ann., 464, 101 (1928).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GRAU, F., HALLIDAY, W. Oxidation and Decarboxylation of Citrate in the Presence of Ferrous Iron. Nature 179, 733–734 (1957). https://doi.org/10.1038/179733a0
Issue Date:
DOI: https://doi.org/10.1038/179733a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.