Abstract
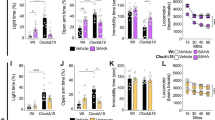
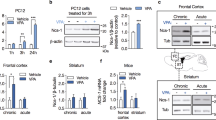
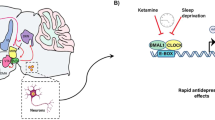
Mice with a mutation in the Clock gene (ClockΔ19) have been identified as a model of mania; however, the mechanisms that underlie this phenotype, and the changes in the brain that are necessary for lithium’s effectiveness on these mice remain unclear. Here, we find that cholecystokinin (Cck) is a direct transcriptional target of CLOCK and levels of Cck are reduced in the ventral tegmental area (VTA) of ClockΔ19 mice. Selective knockdown of Cck expression via RNA interference in the VTA of wild-type mice produces a manic-like phenotype. Moreover, chronic treatment with lithium restores Cck expression to near wild-type and this increase is necessary for the therapeutic actions of lithium. The decrease in Cck expression in the ClockΔ19 mice appears to be due to a lack of interaction with the histone methyltransferase, MLL1, resulting in decreased histone H3K4me3 and gene transcription, an effect reversed by lithium. Human postmortem tissue from bipolar subjects reveals a similar increase in Cck expression in the VTA with mood stabilizer treatment. These studies identify a key role for Cck in the development and treatment of mania, and describe some of the molecular mechanisms by which lithium may act as an effective antimanic agent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kupfer DJ, Angst J, Berk M, Dickerson F, Frangou S, Frank E et al. Advances in bipolar disorder: selected sessions from the 2011 International Conference on Bipolar Disorder. Ann N Y Acad Sci 2011; 1242: 1–25.
McClung CA . Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther 2007; 114: 222–232.
Ko CH, Takahashi JS . Molecular components of the mammalian circadian clock. Hum Mol Genet 2006; 15 Spec No 2: R271–R277.
Lamont EW, Coutu DL, Cermakian N, Boivin DB . Circadian rhythms and clock genes in psychotic disorders. Isr J Psychiatry Relat Sci 2010; 47: 27–35.
Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B et al. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet 2007; 144B: 631–635.
Serretti A, Benedetti F, Mandelli L, Lorenzi C, Pirovano A, Colombo C et al. Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. Am J Med Genet B Neuropsychiatr Genet 2003; 121B: 35–38.
Soria V, Martinez-Amoros E, Escaramis G, Valero J, Perez-Egea R, Garcia C et al. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology 2010; 35: 1279–1289.
Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ et al. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav 2006; 5: 150–157.
Mansour HA, Talkowski ME, Wood J, Chowdari KV, McClain L, Prasad K et al. Association study of 21 circadian genes with bipolar I disorder, schizoaffective disorder, and schizophrenia. Bipolar Disord 2009; 11: 701–710.
Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR . Circadian polymorphisms associated with affective disorders. J Circadian Rhythms 2009; 7: 2.
Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 234–241.
Sjoholm LK, Backlund L, Cheteh EH, Ek IR, Frisen L, Schalling M et al. CRY2 is associated with rapid cycling in bipolar disorder patients. PLoS One 2010; 5: e12632.
Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB . The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci 2007; 9: 333–342.
Harvey AG . Sleep and circadian functioning: critical mechanisms in the mood disorders? Annu Rev Clin Psychol 2011; 7: 297–319.
Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994; 264: 719–725.
McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA 2005; 102: 9377–9381.
Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA 2007; 104: 6406–6411.
Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A et al. Knockdown of clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry 2010; 68: 503–511.
Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology 2011; 36: 1478–1488.
Miyoshi R, Kito S, Nomoto T . Cholecystokinin increases intracellular Ca2+ concentration in cultured striatal neurons. Neuropeptides 1991; 18: 115–119.
Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K . Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature 1980; 285: 476–478.
Lanca AJ, De Cabo C, Arifuzzaman AI, Vaccarino FJ . Cholecystokinergic innervation of nucleus accumbens subregions. Peptides 1998; 19: 859–868.
Ghijsen WE, Leenders AG, Wiegant VM . Regulation of cholecystokinin release from central nerve terminals. Peptides 2001; 22: 1213–1221.
Voigt MM, Wang RY . In vivo release of dopamine in the nucleus accumbens of the rat: modulation by cholecystokinin. Brain Res 1984; 296: 189–193.
Rotzinger S, Lovejoy DA, Tan LA . Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 2010; 31: 736–756.
King DP, Vitaterna MH, Chang AM, Dove WF, Pinto LH, Turek FW et al. The mouse clock mutation behaves as an antimorph and maps within the W(19H) deletion, distal of kit. Genetics 1997; 146: 1049–1060.
Enwright JF 3rd, Wald M, Paddock M, Hoffman E, Arey R, Edwards S et al. DeltaFosB indirectly regulates Cck promoter activity. Brain Res 2010; 1329: 10–20.
Tsankova NM, Kumar A, Nestler EJ . Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci 2004; 24: 5603–5610.
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ . Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 2006; 9: 519–525.
Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ et al. Human postmortem tissue: what quality markers matter? Brain Res 2006; 1123: 1–11.
Ghose S, Crook JM, Bartus CL, Sherman TG, Herman MM, Hyde TM et al. Metabotropic glutamate receptor 2 and 3 gene expression in the human prefrontal cortex and mesencephalon in schizophrenia. Int J Neurosci 2008; 118: 1609–1627.
Hansen TV . Cholecystokinin gene transcription: promoter elements, transcription factors and signaling pathways. Peptides 2001; 22: 1201–1211.
Katada S, Sassone-Corsi P . The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol 2010; 17: 1414–1421.
Weber M, Lauterburg T, Tobler I, Burgunder JM . Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neurosci Lett 2004; 358: 17–20.
Schade R, Vick K, Ott T, Sohr R, Pfister C, Bellach J et al. Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats—influence on dopaminergic stimulation. Chronobiol Int 1995; 12: 87–99.
Hernando F, Fuentes JA, Roques BP, Ruiz-Gayo M . The CCKB receptor antagonist, L-365 260, elicits antidepressant-type effects in the forced-swim test in mice. Eur J Pharmacol 1994; 261: 257–263.
Hughes J, Boden P, Costall B, Domeney A, Kelly E, Horwell DC et al. Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc Natl Acad Sci USA 1990; 87: 6728–6732.
Grandjean EM, Aubry JM . Lithium: updated human knowledge using an evidence-based approach: Part I: Clinical efficacy in bipolar disorder. CNS Drugs 2009; 23: 225–240.
Malhi GS, Adams D, Berk M . Is lithium in a class of its own? a brief profile of its clinical use. Aust N Z J Psychiatry 2009; 43: 1096–1104.
Crump NT, Hazzalin CA, Bowers EM, Alani RM, Cole PA, Mahadevan LC . Dynamic acetylation of all lysine-4 trimethylated histone H3 is evolutionarily conserved and mediated by p300/CBP. Proc Natl Acad Sci USA 2011; 108: 7814–7819.
Guillemette B, Drogaris P, Lin HH, Armstrong H, Hiragami-Hamada K, Imhof A et al. H3 lysine 4 is acetylated at active gene promoters and is regulated by H3 lysine 4 methylation. PLoS Genet 2011; 7: e1001354.
Martin C, Zhang Y . The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 2005; 6: 838–849.
Nightingale KP, Gendreizig S, White DA, Bradbury C, Hollfelder F, Turner BM . Cross-talk between histone modifications in response to histone deacetylase inhibitors: MLL4 links histone H3 acetylation and histone H3K4 methylation. J Biol Chem 2007; 282: 4408–4416.
Doi M, Hirayama J, Sassone-Corsi P . Circadian regulator CLOCK is a histone acetyltransferase. Cell 2006; 125: 497–508.
Zhao WN, Malinin N, Yang FC, Staknis D, Gekakis N, Maier B et al. CIPC is a mammalian circadian clock protein without invertebrate homologues. Nat Cell Biol 2007; 9: 268–275.
Moretti M, Valvassori SS, Varela RB, Ferreira CL, Rochi N, Benedet J et al. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav Pharmacol 2011; 22: 766–772.
Acknowledgements
We are grateful to Dr Joe Takahashi for the ClockΔ19 mice. We thank Elizabeth Gordon and Ariel Ketcherside for assistance with mouse husbandry and genotyping, Dr Shari Birnbaum and Ami Petterson for assistance with behavioral testing, and Dr Shibani Mukherjee for assistance with AAV construction and design. We also thank Dr Syann Lee and Dr Joel Elmquist for assistance with laser capture equipment. We also thank Dr R Jude Samulski and the UNC Gene Therapy Vector Core for assistance with AAV preparation. This study was funded by The McKnight Endowment Fund for Neuroscience, The Brain & Behavior Research Foundation (NARSAD) and the NIMH (MH082876).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Arey, R., Enwright, J., Spencer, S. et al. An important role for Cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors. Mol Psychiatry 19, 342–350 (2014). https://doi.org/10.1038/mp.2013.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.12
Keywords
This article is cited by
-
Microarray evidence that 8-cell human embryos express some hormone family members including oxytocin
Journal of Assisted Reproduction and Genetics (2024)
-
Mood and behavior regulation: interaction of lithium and dopaminergic system
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
The trilateral interactions between mammalian target of rapamycin (mTOR) signaling, the circadian clock, and psychiatric disorders: an emerging model
Translational Psychiatry (2022)
-
Valproate reverses mania-like behaviors in mice via preferential targeting of HDAC2
Molecular Psychiatry (2021)
-
Mesocortical BDNF signaling mediates antidepressive-like effects of lithium
Neuropsychopharmacology (2020)