Abstract
LGALS3, a member of the lectin family, has an important role in tumor progression through inhibition of apoptosis. LGALS3 shares several significant structural properties with BCL2. In this study, we examined the prognostic significance of LGALS3 and BCL2 in uniformly treated classical Hodgkin’s lymphoma. Diagnostic tissues from 110 patients with uniformly treated classical Hodgkin’s lymphoma were evaluated retrospectively by immunohistochemical analysis of LGALS3 and BCL2 expression. The median follow-up time was 6.2 years (range, 0.2–17.3 years). Twenty-seven patients (25%) expressed LGALS3 protein in Hodgkin/Reed–Sternberg cells, which was associated with poor overall survival and event-free survival (P=0.007 and P<0.001). Fifteen patients (14%) expressed BCL2 protein in Hodgkin/Reed–Sternberg cells, which was not associated with overall survival and event-free survival (P=0.928 and P=0.900).There was no correlation between LGALS3 and BCL2 expression (P=0.193). Multivariate analysis identified LGALS3 protein as an independent prognostic factor for event-free survival (P=0.007). Subgroup analysis according to the Ann Arbor stage of classical Hodgkin’s lymphoma showed that LGALS3 protein expression had a prognostic value in limited-stage classical Hodgkin’s lymphoma (P<0.001). The results of this study suggest that LGALS3 is an independent prognostic factor in classical Hodgkin’s lymphoma, and may allow the identification of a subgroup of patients with limited-stage classical Hodgkin’s lymphoma who require more intensive therapy.
Similar content being viewed by others
Main
Classical Hodgkin’s lymphoma is a highly curable lymphoid malignancy.1 However, despite important treatment advances, a minority of patients do not respond to therapy or relapse after optimal initial therapeutic strategy, and may require adapted first-line treatment.2 While the International Prognostic Score is useful in clinical practice, it does not fully reflect the biological behavior of classical Hodgkin’s lymphoma.3
Galectins, a family of mammalian β-galactoside-binding lectin molecules, are implicated in inflammation and tumor progression.4 LGALS3 is expressed in lymphoma and myeloma cells,5, 6 and has both pro-apoptotic and antiapoptotic functions.7 A recent study demonstrated that high expression of LGALS3 protein in diffuse large B-cell lymphoma cells was associated with resistance to apoptosis.6 Moreover, LGALS3 has an important role in angiogenesis and metastases in several malignancies, including B-cell lymphomas.7, 8, 9
B-cell lymphoma-2 (BCL2) protein inhibits apoptosis by preventing cytochrome release from mitochondria, thus blocking oxidative cell destruction. Over-expression of BCL2 has been found in some solid malignancies and malignant lymphomas including classical Hodgkin’s lymphoma.10, 11, 12, 13 The BCL2 family of antiapoptotic proteins contribute to the survival of Hodgkin/Reed–Sternberg cells by counteracting the expression of the pro-apoptotic proteins bax, and bad.13 There is specific sequence homology between the NWGR (Asp–Trp–Gly–Arg) amino acid sequence in LGALS3 and the BH1 domain of the BCL2 gene family.14 Furthermore, LGALS3/BCL2 heterodimerization promotes the BCL2 antiapoptotic effect.14
Although there is evidence that LGALS3 and BCL2 are associated with tumor progression in hematologic malignancies including B-cell lymphomas,14, 15 no study has examined the relationship between LGALS3 and BCL2, and their prognostic implication in classical Hodgkin’s lymphoma patients. This retrospective study evaluated LGALS3 and BCL2 expression in classical Hodgkin’s lymphoma patients and examined the correlations between these markers and their prognostic significance.
Materials and methods
Patients
This retrospective study reviewed histological and immunohistochemical data from 110 consecutive patients diagnosed with classical Hodgkin’s lymphoma at Asan Medical Center, Seoul, South Korea, between 1990 and 2012. All patients had pathologically confirmed classical Hodgkin’s lymphoma, treated with doxorubicin, bleomycin, vinblastine, and dacarbazine therapy regimen, with or without radiation. The patients were aged ≥15 years at diagnosis, and had no previous treatment and no history of malignancy. All samples were obtained before to treatment. Follow-up data were available for all patients included in the study.
The median follow-up time was 6.2 years (range, 0.2–17.3 years). Response criteria were based on standard guidelines. Routine follow-up imaging analyses were performed every 3 months for the first 2 years, every 6 months for the next 3 years, and annually (or whenever clinically indicated) thereafter. We defined the limited-stage as Ann Arbor stage I–II without B symptom or bulky disease.
The present research was approved by the Internal Review Board of the Asan Medical Center.
Histopathological Analysis and Immunohistochemistry
All histological and immunophenotypic data were reviewed by three pathologists (JH, YWK, and SJJ). According to the World Health Organization criteria, the classical Hodgkin’s lymphoma cases were subtyped as follows: nodular sclerosis, lymphocyte-rich, mixed cellularity, lymphocyte-depleted, or not otherwise specified classical Hodgkin’s lymphoma.16 Tissue microarray was constructed with three 1 mm diameter tumor cores from selected areas of formalin-fixed, paraffin-embedded tumor samples. The tissue microarray section was stained using an automatic immunohistochemistry staining device (Benchmark XT, Ventana Medical System, Tucson, AZ, USA). Briefly, 5 μm thick sections were transferred onto poly-L-lysine-coated adhesive slides and dried at 62 °C for 30 min. After standard heat epitope retrieval for 30 min in ethylenediamine tetraacetic acid (pH 8.0), the samples were incubated with antibodies against cleaved LGALS3 (dilution 1:200; NOVO, Newcastle, UK), and BCL2 (dilution 1:50; Cell Marque, Rocklin, CA, USA). The sections were then incubated with biotinylated antimouse immunoglobulins, peroxidase-conjugated streptavidin (LSAB kit, DAKO, Glostrup, Denmark), and 3,3′-diaminobenzidine. Slides were counterstained with Harris hematoxylin.
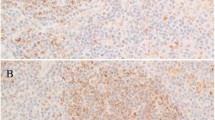
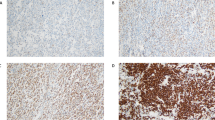
In the tissue microarray, each case was represented by three tissue cores, and at least ten CD30-positive Hodgkin/Reed–Sternberg cells, in at least one of the three core cylinders from each patient, were read. A sample was considered LGALS3- or BCL2-positive if the expression of these markers was detected in ≥10% of Hodgkin/Reed–Sternberg cells (Figures 1a and b). A sample was considered LGALS3- or BCL2-negative if LGALS3 or BCL2 expression was detected in <10% of the Hodgkin/Reed–Sternberg cells, or in bystander cells only.
In situ hybridization analysis of Epstein-Barr Virus (EBV)-encoded RNA-1 and RNA-2 was performed and scored as previously described.17
Statistical Analysis
Overall survival was defined as the interval between the date of diagnosis and death from any cause. The follow-up of living patients was censored at their last follow-up date. Event-free survival was defined as the interval between the date of diagnosis and the date of disease progression, relapse, or death from any cause. Cumulative overall survival and event-free survival were analyzed by the Kaplan–Meier method, and comparisons were performed by log-rank testing.
Multivariate prognostic analyses of overall survival and event-free survival were performed with the Cox proportional hazards regression model using the enter method. Categorical variables were compared using the χ2-test. All statistical analyses were performed using the SPSS statistical software program (version 18.0; SPSS, Chicago, IL, USA) or R 2.15.2. All P-values are two-sided associations and P<0.05 was considered statistically significant.
Results
Patient Characteristics
The clinical characteristics of the 110 patients included in the study are summarized in Table 1. The median age of the patients was 35 years (range, 15–77 years). Forty-two patients experienced relapse, disease progression, or death, and 20 patients died. Median overall survival and event-free survival were not reached. The estimated 5-year overall survival and event-free survival were 83 and 58%, respectively.
LGALS3 and BCL2 Expression in Classical Hodgkin’s Lymphoma Tissues
The correlations between LGALS3, BCL2, and clinical variables are summarized in Supplementary Table 1.The expression of LGALS3 or BCL2 was not associated with clinical variables. There was no correlation between LGALS3 and EBV status (P>0.999) and between BCL2 and EBV status (P=0.162).There was no correlation between LGALS3 and BCL2 expression (P=0.193).
Prognostic Significance of LGALS3 and BCL2 Expression
Patients with LGALS3 expression had lower 5-year overall-survival rates (73 vs 88%, P=0.007; Figure 2a), and lower 5-year event-free survival rates (44 vs 67%, P<0.001; Figure 2b) than patients with no LGALS3 expression. BCL2 expression was not significantly associated with either overall survival or event-free survival (P=0.928, Figure 2c; and P=0.900, Figure 2d, respectively).
Univariate analysis revealed that both overall survival and event-free survival were associated with the International Prognostic Score (≥4). Multivariate analysis identified LGALS3 index as an independent prognostic marker for event-free survival (P=0.007, Table 2), along with high-risk International Prognostic Score (≥4), and a marginal prognostic marker for overall survival (P=0.063, Table 2).
As the International Prognostic Score is a significant prognostic factor only in advanced-stage disease,3 we performed a subgroup analysis according to the disease stage, to determine whether LGALS3 may have an advantage over the International Prognostic Score. In limited-stage classical Hodgkin’s lymphoma cases, high LGALS3 expression was associated with worse overall-survival rate compared with low LGALS3 expression, (P<0.001; Figure 3a). In patients with advanced-stage classical Hodgkin’s lymphoma, LGALS3 positivity was not associated with overall survival (P=0.189; Figure 3b).
Comparison of survival rates according to LGALS3 expression and Ann Arbor stage of classical Hodgkin’s lymphoma. LGALS3 positivity in patients with limited-stage classical Hodgkin’s lymphoma was associated with worse overall survival (a). In patients with advanced-stage classical Hodgkin’s lymphoma, LGALS3 positivity was not associated with overall survival (b). Comparison of survival rates according to International Prognostic Score and Ann Arbor stage. Patients with high-risk International Prognostic Score was not associated with worse overall survival (c) in patients with limited-stage classical Hodgkin’s lymphoma, however, high-risk International Prognostic Score was associated with overall survival (d) in advanced-stage classical Hodgkin’s lymphoma.
By contrast, the International Prognostic Score was not significantly associated with overall survival in limited-stage classical Hodgkin’s lymphoma (P=0.468; Figure 3c), although, it was significantly associated with overall survival in advanced-stage classical Hodgkin’s lymphoma cases (P<0.001; Figure 3d).
Discussion
This study is the first to evaluate the prognostic value of LGALS3 expression in classical Hodgkin’s lymphoma patients. In a cohort of 110 patients with ABVD-treated classical Hodgkin’s lymphoma, LGALS3 expression had a prognostic value in limited-stage classical Hodgkin’s lymphoma. However, BCL2 expression was not associated with LGALS3 expression and had no prognostic value.
Intracellular LGALS3 protects different cell types against apoptosis in response to various stimuli.7 Thus, LGALS3-transfected Jurkat cells become resistant to apoptosis induced by anti-Fas antibody and staurosporine, compared with controls.18 Likewise, Burkitt lymphoma cells transfected with a LGALS3-expressing plasmid show increased resistance to anti-Fas-induced cell death.8 On the other hand, secreted extracellular LGALS3 molecules induce T-cell apoptosis, thereby having an important role in the immune escape during tumor progression, through induction of apoptosis of cancer-infiltrating T-cells.19 We found that intracellular LGALS3 was associated with poor prognosis in classical Hodgkin’s lymphoma patients, in agreement with previous studies of LGALS3 in solid tumors and other lymphomas.7, 8, 18
In contrast to LGALS3, BCL2 expression was not associated with overall survival or event-free survival. Although LGALS3 is not a member of the BCL2 gene family, it shares several significant structural properties with BCL2.14 The antiapoptotic activity of LGALS3 is mediated, at least in part, by the activation of ERK and JNK (c-Jun NH2-terminal kinase) pathway20 or CD95 (APO-1/Fas) receptor, a member of the death receptor family.21 Further studies are needed to determine the precise mechanism of LGALS3-mediated antiapoptosis in classical Hodgkin’s lymphoma patients.
In addition to its antiapoptotic effect, LGALS3 has a role in cell growth, cell adhesion, cell differentiation, and tumor progression and metastasis, mainly through binding to glycoproteins.22, 23, 24 LGALS3 stimulates capillary tube formation by human umbilical vein endothelial cells in vitro, and angiogenesis in vivo.25 Furthermore, LGALS3 increases α4 and β7 integrin expression in the breast cancer cell line Evsa-T and promotes Evsa-T adhesion to laminin, fibronectin, and vitronectin,26 which suggests that LGALS3 may induce metastasis by specifically influencing cell adhesion to the extracellular matrix. Further studies are needed to delineate the mechanism of LGALS3-mediated adverse effects on the prognosis of classical Hodgkin’s lymphoma patients.
EBV, a ubiquitous herpes virus that infects more than 90% of humans, is now classified as a group 1 human carcinogen by the International Agency for Research on Cancer owing to its etiologic role in classical Hodgkin’s lymphoma.27Our study found no correlation between LGALS3 expression and EBV status. These results suggest that LGALS3 expression was associated with survival outcome independently of EBV status.
In the present study, LGALS3 expression was significantly associated with poor prognosis, especially for patients with limited-stage disease. Although the International Prognostic Score is the standard stratification system for survival in patients with advanced-stage disease, it fails to stratify the subgroups with worse prognosis in patients with limited-stage classical Hodgkin’s lymphoma.3 Our results also revealed that the International Prognostic Score was associated with prognosis only in advanced-stage classical Hodgkin’s lymphoma. Therefore, LGALS3 expression is a viable prognostic factor alternative to the International Prognostic Score in limited-stage classical Hodgkin’s lymphoma.
Studies assessing the prognostic significance of BCL2 expression in patients with classical Hodgkin’s lymphoma have yielded conflicting results. Although several reports found a significantly negative prognostic effect of its over-expression in Hodgkin/Reed–Sternberg cells,28, 29, 30, 31, 32, 33 other studies found no impact.34, 35, 36 Possible reasons for the inter-study discrepancy include disparate study populations, technical differences, for example, different antibody clones, use of tissue microarray vs whole sections, inter-observer variability, different criteria of BCL2 positivity, and disparate use of the index of outcome. For example, Sanchez-Espiridion et al33 used a population with advanced-stage patients, whereas we studied a series of consecutive patients in one hospital. While the chemotherapy regimens in some previous studies were varied,29, 30 we limited our analysis to patients treated with a doxorubicin, bleomycin, vinblastine, and dacarbazine regimen.
The limitations of this study include its retrospective design, short follow-up period of some recent cases, small sample size, and tissue microarray-based design. Tissue microarray design cannot reflect the entire distribution of tumor because of tissue heterogeneity.
In summary, our results suggest that LGALS3 is an independent prognostic factor in classical Hodgkin’s lymphoma, and may be useful in the identification of a subgroup of patients with limited-stage classical Hodgkin’s lymphoma who are at a high risk for recurrence or progression and who may benefit from aggressive chemotherapy. Further studies, including prospective clinical trials, are needed to confirm the present findings, and investigate the effects of LGALS3 expression on clinical outcomes.
References
Evens AM, Hutchings M, Diehl V . Treatment of Hodgkin lymphoma: the past, present, and future. Nat Clin Pract Oncol 2008;5:543–556.
Quddus F, Armitage JO . Salvage therapy for Hodgkin's lymphoma. Cancer J 2009;15:161–163.
Hasenclever D, Diehl V . A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on advanced Hodgkin's Disease. N Engl J Med 1998;339:1506–1514.
Giordano M, Croci DO, Rabinovich GA . Galectins in hematological malignancies. Curr Opin Hematol 2013;20:327–335.
Streetly MJ, Maharaj L, Joel S et al. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood 2010;115:3939–3948.
Clark MC, Pang M, Hsu DK et al. Galectin-3 binds to CD45 on diffuse large B-cell lymphoma cells to regulate susceptibility to cell death. Blood 2012;120:4635–4644.
Dumic J, Dabelic S, Flogel M . Galectin-3: an open-ended story. Biochim Biophys Acta 2006;1760:616–635.
Hoyer KK, Pang M, Gui D et al. An antiapoptotic role for galectin-3 in diffuse large B-cell lymphomas. Am J Pathol 2004;164:893–902.
Nakahara S, Oka N, Raz A . On the role of galectin-3 in cancer apoptosis. Apoptosis 2005;10:267–275.
Reed JC, Miyashita T, Takayama S et al. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem 1996;60:23–32.
Martinez-Arribas F, Alvarez T, Del Val G et al. Bcl-2 expression in breast cancer: a comparative study at the mRNA and protein level. Anticancer Res 2007;27:219–222.
Leahy DT, Mulcahy HE, O'Donoghue DP et al. bcl-2 protein expression is associated with better prognosis in colorectal cancer. Histopathology 1999;35:360–367.
Bai M, Papoudou-Bai A, Horianopoulos N et al. Expression of bcl2 family proteins and active caspase 3 in classical Hodgkin's lymphomas. Hum Pathol 2007;38:103–113.
Akahani S, Nangia-Makker P, Inohara H et al. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res 1997;57:5272–5276.
Cheng YL, Huang WC, Chen CL et al. Increased galectin-3 facilitates leukemia cell survival from apoptotic stimuli. Biochem Biophys Res Commun 2011;412:334–340.
Swerdlow SH, Campo E, Harris NL et al. World Health Organization classification of tumours of haematopoietic and lymphoid tissues 2008, IARC Press: Lyon, France, pp 326–334.
Huh J, Cho K, Heo DS et al. Detection of Epstein-Barr virus in Korean peripheral T-cell lymphoma. Am J Hematol 1999;60:205–214.
Yang RY, Hsu DK, Liu FT . Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA 1996;93:6737–6742.
Fukumori T, Takenaka Y, Yoshii T et al. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res 2003;63:8302–8311.
Takenaka Y, Fukumori T, Yoshii T et al. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol 2004;24:4395–4406.
Fukumori T, Takenaka Y, Oka N et al. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res 2004;64:3376–3379.
Rabinovich GA, Baum LG, Tinari N et al. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol 2002;23:313–320.
Liu FT, Patterson RJ, Wang JL . Intracellular functions of galectins. Biochim Biophys Acta 2002;1572:263–273.
Takenaka Y, Fukumori T, Raz A . Galectin-3 and metastasis. Glycoconj J 2004;19:543–549.
Nangia-Makker P, Honjo Y, Sarvis R et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol 2000;156:899–909.
Matarrese P, Fusco O, Tinari N et al. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer 2000;85:545–554.
Bouvard V, Baan R, Straif K et al. A review of human carcinogens-Part B: biological agents. Lancet Oncol 2009;10:321–322.
Rassidakis GZ, Medeiros LJ, Vassilakopoulos TP et al. BCL-2 expression in Hodgkin and Reed-Sternberg cells of classical Hodgkin disease predicts a poorer prognosis in patients treated with ABVD or equivalent regimens. Blood 2002;100:3935–3941.
Canioni D, Deau-Fischer B, Taupin P et al. Prognostic significance of new immunohistochemical markers in refractory classical Hodgkin lymphoma: a study of 59 cases. PLoS One 2009;4:e6341.
Garcia JF, Camacho FI, Morente M et al. Hodgkin and Reed-Sternberg cells harbor alterations in the major tumor suppressor pathways and cell-cycle checkpoints: analyses using tissue microarrays. Blood 2003;101:681–689.
Sup SJ, Alemany CA, Pohlman B et al. Expression of bcl-2 in classical Hodgkin's lymphoma: an independent predictor of poor outcome. J Clin Oncol 2005;23:3773–3779.
Jakovic LR, Mihaljevic BS, Jovanovic MD et al. Prognostic significance of Bcl-2, tumor-associated macrophages, and total neoplastic and inflammatory lymph node involvement in advanced stage classical Hodgkin’s lymphoma. Onkologie 2012;35:733–739.
Sanchez-Espiridion B, Montalban C, Lopez A et al. A molecular risk score based on 4 functional pathways for advanced classical Hodgkin lymphoma. Blood 2010;116:e12–e17.
Montalban C, Garcia JF, Abraira V et al. Influence of biologic markers on the outcome of Hodgkin's lymphoma: a study by the Spanish Hodgkin's Lymphoma Study Group. J Clin Oncol 2004;22:1664–1673.
Morente MM, Piris MA, Abraira V et al. Adverse clinical outcome in Hodgkin's disease is associated with loss of retinoblastoma protein expression, high Ki67 proliferation index, and absence of Epstein-Barr virus-latent membrane protein 1 expression. Blood 1997;90:2429–2436.
Spector N, Milito CB, Biasoli I et al. The prognostic value of the expression of Bcl-2, p53 and LMP-1 in patients with Hodgkin's lymphoma. Leuk Lymphoma 2005;46:1301–1306.
Acknowledgements
This study was supported by a Grant (2011-090) from the Asan Institute for Life Sciences, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Koh, Y., Jung, S., Park, CS. et al. LGALS3 as a prognostic factor for classical Hodgkin’s lymphoma. Mod Pathol 27, 1338–1344 (2014). https://doi.org/10.1038/modpathol.2014.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2014.38
Keywords
This article is cited by
-
E2F5 Targeted by Let-7d-5p Facilitates Cell Proliferation, Metastasis and Immune Escape in Gallbladder Cancer
Digestive Diseases and Sciences (2024)