Abstract
Pilomatricoma, also known as ‘calcifying epithelioma of Malherbe’, is a common skin adnexal tumor that mimics hair growth. Its proliferating cells seem distinctly programmed to undergo terminal differentiation and death. We report the first cytogenetic investigations of pilomatricoma. Trisomy 18 was shown, in an index case, by G-banded karyotyping. This aberration was corroborated by interphase fluorescence in situ hybridization, using a chromosome 18 pericentromeric probe, in the basaloid epithelial component of 7 of 11 pilomatricomas, including the index case. Trisomy 18 was present in a small subset of cells, suggesting a role in pilomatricoma progression, rather than in tumor initiation. We conclude that trisomy 18 is a consistent feature in pilomatricoma, suggesting that genes carried on this chromosome, such as that for the antiapoptotic oncoprotein BCL2, may have a role in the growth and differentiation of this benign self-limited tumor.
Similar content being viewed by others
Main
Pilomatricoma, a skin adnexal tumor that recapitulates hair growth,1, 2, 3 is by far the most common epithelial neoplasm of childhood.4 The proliferating cells in pilomatricoma seem distinctly programmed to undergo terminal differentiation and death, which is perhaps responsible for the tumor's inevitably benign clinical course. The cytogenetic features of pilomatricoma have not been explored. We analyze a series of pilomatricomas for the presence and cellular localization of trisomy 18, based on our finding of trisomy 18 in an index case.
Materials and methods
We performed cytogenetic analysis on tissue from an otherwise-healthy 12-year-old girl with an enlarging subcutaneous right neck mass. Fresh samples of tissue were processed for short-term adherent cultures (4–10 days), harvested in situ for cytogenetic analysis, and stained by G-banding according to standard procedures.
All other cases diagnosed as pilomatricoma over a 9-month period were retrieved from the pathology department files at Children's Hospital Boston (Boston, MA, USA). In addition to the index case, 10 cases with a substantial basaloid cell component were selected for study. Fluorescence in situ hybridization (FISH) was performed on formalin-fixed, paraffin-embedded, 5 μm-thick tissue sections as previously described.5 A total of 40–48 min proteolytic digestion time was used (pepsin solution, Zymed, DIGEST-ALL 3, No. 00-3009), according to the status of each section. Chromosome 18 FISH assay was performed using an α-satellite centromeric probe from Abbott Molecular (CEP D18Z1, No. 05J10-018). The number of fluorescence signals per nucleus was counted in at least 100 cells from various areas of viable tumor and from surrounding mesenchymal or epithelial tissue for comparison.
Results
Cytogenetic Analysis
Pilomatricoma cytogenetic analysis from the 12-year-old index patient revealed that 2 of 19 metaphase cells from independent 7-day adherent cultures showed trisomy 18 with the following karyotype: 47, XX, +18[2] (Figure 1). The remaining 17 cells had a normal karyotype with no structural or numerical chromosomal abnormalities.
Clinical Features
Including the index case, the 11 patients ranged in age from 2 to 17 years (mean 8 years; median 7 years). Of these patients, two were male and nine were female. Affected sites included the skin/subcutis of the brow, temple, eyelid, cheek, neck (n=4), chest (n=2), and forearm. The pilomatricomas occurred singly in all patients except for a 2-year-old girl with autism who developed a contralateral facial pilomatricoma 6 months later. Other underlying medical conditions included a history of supraventricular tachycardia treated by ablation as well as a history of left ankle osteochondritis dissecans, both in a 17-year-old girl, and previously repaired hiatal hernia and gastric volvulus in a 2-year-old girl. No associated medical conditions were noted in any of the other eight patients.
Pathologic Findings
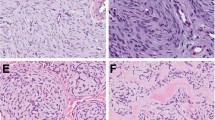
All lesions were tan and chalky grossly, and ranged in size from 1.0 to 3.8 cm. Microscopically, all showed multi-layered basaloid epithelium with viable nuclei as well as eosinophilic, cornified material consistent with ‘shadow cells’ (Figure 2a). Calcification and multinucleate giant cells were also present in all cases.
(a) All pilomatricomas showed basaloid epithelial cells in a maturational continuum with senescent anucleate ‘shadow cells’ (original magnification × 400). (b) Fluorescent in situ hybridization using a centromeric probe for chromosome 18 showed cells with three signals, indicating a subset of tumor cells with trisomy 18 (original magnification × 1000).
FISH Analysis
FISH with a centromeric probe for chromosome 18 on 5-μm thick formalin-fixed paraffin-embedded sections confirmed trisomy 18 in 11 of 400 cells (2.8%) from the index case (Figure 2b), which is above the normal range of 0–0.2%. This abnormality was confined to the epithelial component of the tissue. Out of the 10 additional cases studied, 6 similarly featured trisomy 18 in 0.5–1.5% of neoplastic cells. Overall, 7 out of 11 cases showed trisomy 18 in 0.5–2.8% of neoplastic cells (mean 1.1%; s.d. 0.83%). Trisomy 18 was not observed in the surrounding mesenchymal or epithelial tissue in any of the 11 cases.
Discussion
We report the first cytogenetic alteration in pilomatricoma: trisomy 18. Our study shows this aberration to be a consistent finding in pilomatricoma, although occurring in only a subset of the proliferating tumor cells.
In this study, trisomy 18 was confined to the neoplastic epithelial cells of pilomatricoma. The lineage involvement of clonal cytogenetic aberrations is of increasing interest in various tumor types, with some tumorigenic abnormalities in epithelial tumors being restricted to the neoplastic epithelial components, and others to the apparently non-neoplastic stroma.6 Although trisomy 18 was a recurrent finding, observed in 7 of 11 pilomatricomas in this study, the aberration was shown in a minority of the presumed neoplastic cells. These findings suggest that trisomy 18 is not the initiating oncogenic event in pilomatricoma; but, it is also possible that percentage of such trisomic cells was underestimated. In the karyotyping study of the index case, preferential culture of the stromal cells or non-neoplastic epithelial cells could be a cause. In the FISH analysis, the sectioning artifact, at 5 μm, would have certainly contributed to an underestimate of trisomies. The senescence and dropout of a subset of nuclei (in the progression from basaloid cells to ‘shadow cells’) is likely a further contributing factor. Although these data suggest that trisomy 18 is not the initiating mutation, it is nonetheless possible that trisomy 18 does have a biologic role, although in a subset of cells, that induces neoplastic growth, differentiation, or senescence in neighboring epithelial cells by paracrine signaling or direct cell-to-cell interactions. Interactions with neighboring stromal cells are also conceivable, particularly in light of the trisomic cells’ localization at the periphery of the lesions. These possibilities remain to be investigated.
Previous studies have suggested a role for the BCL-2 oncoprotein in the oncogenesis of pilomatricomas. BCL-2 is found on chromosome 18, and in one series it was shown to be overexpressed by immunohistochemical analysis in all 10 pilomatricomas, although on a greater proportion of cells than show trisomy 18.7 BCL-2 is an antiapoptotic regulator that is involved in the highly regulated growth cycle of hair follicles from anagen phase (growth) to catagen phase (regression) to telogen phase (resting). Overexpression of BCL-2 oncoprotein has been shown in a wide variety of tumors, including follicular lymphoma, the B-cell lymphoma for which it was originally named. Our findings give further support to the role of BCL-2 in the development of pilomatricomas.
Previous studies have also implicated the WNT/β-catenin signaling pathway in the oncogenesis of pilomatricomas, with β-catenin mutations found in approximately 75% of cases.8 In accordance with this finding, pilomatricomas are known to occur in conjunction with Gardner's syndrome, a dominant hereditary genetic syndrome characterized by a germline mutation in a single APC gene allele, which is a well-described suppressor of the WNT/β-catenin signaling pathway. Future studies will be needed to determine the interaction between these oncogenic signaling pathways.
To summarize, we have shown trisomy 18 as a consistent feature in pilomatricoma, occurring in the basaloid epithelial cell component. The genetic mechanisms involved in the biology of pilomatricoma are of particular interest because they seem geared to produce cell senescence and death, features that would be highly desirable to achieve in the treatment of more aggressive types of epithelial neoplasia.
References
Ackerman AB, Reddy VB, Soyer HP, eds. Neoplasms with Follicular Differentiation. Ardor Scribendi: New York, 2001.
Duflo S, Nicollas R, Roman S, et al. Pilomatrixoma of the head and neck in children: a study of 38 cases and a review of the literature. Arch Otolaryngol Head Neck Surg 1998;124:1239–1242.
Kaddu S, Soyer HP, Hödl S, et al. Morphological stages of pilomatricoma. Am J Dermatopathol 1996;18:333–338.
Lucas A, Betlloch I, Planelles M, et al. Non-melanocytic benign skin tumors in children. Am J Clin Dermatol 2007;8:365–369.
Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 2008;216:64–74.
Eng C, Leone G, Orloff MS, et al. Genomic alterations in tumor stroma. Cancer Res 2009;69:6759–6764.
Farrier S, Morgan M . Bcl-2 expression in pilomatricoma. Am J Dermatopathol 1997;19:254–257.
Chan EF, Gat U, McNiff JM, et al. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet 1999;21:410–413.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Agoston, A., Liang, CW., Richkind, K. et al. Trisomy 18 is a consistent cytogenetic feature in pilomatricoma. Mod Pathol 23, 1147–1150 (2010). https://doi.org/10.1038/modpathol.2010.99
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.99