Abstract
Angiomatoid fibrous histiocytoma is a mesenchymal neoplasm of intermediate malignancy and uncertain histogenesis/line of differentiation, which occurs most commonly in the extremities of children to young adults. It has a characteristic appearance characterized by a proliferation of histiocytoid cells with a lymphoid cuff and fibrous pseudocapsule, simulating the appearance of a neoplasm occurring within a lymph node. However, these classic histological features are not always present. Given the variable appearance of the neoplastic cells and the lack of consistently positive immunohistochemical markers, diagnosis can be problematic. Angiomatoid fibrous histiocytoma has been found to harbor three related translocations, a t(12;16)(q13;p11) resulting in a FUS/ATF1 fusion gene, t(12;22)(q13;q12) resulting in a EWSR1/ATF1 fusion, and t(2;22)(q33;q12) resulting in a EWSR1/CREB1 fusion. Fluorescence in situ hybridization (FISH) probes to EWSR1 and FUS, in theory, should detect all three translocations/gene fusions. We evaluated 18 cases of angiomatoid fibrous histiocytoma for rearrangements of EWSR1 and FUS by FISH, the largest series to date. We found that 13 of 17 (76%) cases of angiomatoid fibrous histiocytoma harbored rearrangements of EWSR1; rearrangements of FUS were not detected in any of the cases. This study affirms that the rearrangement of EWSR1 is a common genetic event in angiomatoid fibrous histiocytoma, and is thus useful diagnostically. This study supports the fact that the rearrangement of FUS is present in only a small minority of angiomatoid fibrous histiocytomas. Interestingly, 24% of the cases were translocation negative, and did not contain rearrangements of EWSR1 or FUS by FISH. Although it is possible that these cases contained cryptic rearrangements of EWSR1 or FUS that were not detectable by our FISH probes, it also raises the possibility that another translocation/gene fusion may be present in angiomatoid fibrous histiocytoma. Finally, we discuss some of the potential pitfalls of this technique, including confusion with other mesenchymal neoplasms containing rearrangement of EWSR1, in particular Ewing's sarcoma/PNET.
Similar content being viewed by others
Main
Angiomatoid fibrous histiocytoma is a mesenchymal neoplasm of intermediate malignancy that generally affects children and young adults. It occurs most commonly within the extremities, followed by the trunk as well as the head and neck. Although generally indolent in its clinical behavior, a small but significant number of angiomatoid fibrous histiocytomas recur locally, and rare cases have been known to metastasize.1, 2, 3
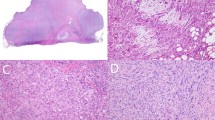
When all of the classic histological features are present, the diagnosis is usually straightforward. This is largely dependent on the recognition of a proliferation of relatively bland histiocytoid cells associated with a lymphoid cuff and fibrous capsule at its periphery, simulating the appearance of a lymph node metastasis (Figure 1a).1, 2, 3 However, this classic appearance is variably present, or may not be sampled in sections submitted for histological analysis. This problem is compounded by the protean appearance of the neoplastic cells and the lack of a consistently positive immunohistochemical marker, making the diagnosis problematic in some cases. Therefore, additional ancillary tests may be helpful in the diagnosis of this neoplasm.
The molecular genetics of angiomatoid fibrous histiocytoma have become increasingly understood, with a number of reports describing the EWSR1/CREB1, EWSR1/ATF1, and FUS/ATF1 gene fusions.4, 5, 6, 7, 8, 9, 10, 11 Fluorescence in situ hybridization (FISH) for EWSR1 and FUS is widely available and is used routinely in medical centers that encounter large numbers of sarcomas/mesenchymal neoplasms. Probes to these two genes should in theory, be able to detect all three translocations/gene fusions reported in angiomatoid fibrous histiocytomas. We hypothesized that rearrangements of EWSR1 and FUS could be detected by FISH in most, if not all, angiomatoid fibrous histiocytomas. We evaluated the utility of EWSR1 and FUS FISH as an adjunct in the diagnosis of angiomatoid fibrous histiocytoma, and compared the relative frequency of rearrangement of these two genes in a series of 18 cases in the largest series of angiomatoid fibrous histiocytomas evaluated for cytogenetic abnormalities.
Materials and methods
Four index cases of angiomatoid fibrous histiocytoma (including consultation material from JRG) were identified at our institution, which harbored rearrangements of EWSR1. The Cleveland Clinic Anatomic Pathology database was searched for the diagnosis of angiomatoid fibrous histiocytoma; two cases for which paraffin blocks were available were added. An additional 12 cases were identified from personal consultation material of two of the authors (BPR and EM). The morphological diagnosis of these cases was confirmed by at least two pathologists with expertise in soft tissue pathology. Standard immunohistochemical studies were reviewed or conducted using the following conditions: desmin (D33, 1:10; DAKO, Carpinteria, CA, USA), muscle-specific actin (HHF35, 1:40; ENZO, Plymouth Meeting, PA, USA), smooth muscle actin (1A-4, 1:50; DAKO), pan-cytokeratin (AE1/AE3, 1:200; Roche/Chemicon), EMA (E29, 1:50; DAKO) S-100 protein (polyclonal 1:200; DAKO), and CD99 (H036-1.1, prediluted; Ventana, Tucson, AZ, USA).
FISH studies were carried out on interphase nuclei present on formalin-fixed paraffin-embedded tissue sections as reported previously.12 Detection of rearrangement of EWSR1 and FUS was performed with EWSR1 (22q12) and LSI FUS (16p11) Dual Color, Break Apart Rearrangement Probes (Abbott Molecular/Vysis, Des Plaines, IL, USA).
Results
The group comprised 7 males and 10 females (the gender of one patient was not known) with an age range of 1–47 years (mean: 22 years). The most common sites included the upper extremities (8), followed by the head and neck (3), trunk (3), lower extremities (3), and omentum (1).
By immunochemistry, desmin was positive in (12/13) 92% of the cases. However, three of the cases were only focally positive. Immunoreactivity for CD68 was seen in 67% (4/6), CD99 in 80% (4/5), EMA in 50% (4/8), and SMA in 38% (3/8) of cases (Table 1).
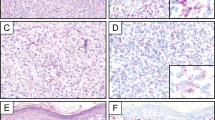
FISH identified a rearrangement of the EWSR1 locus in 76% of cases (13/17); one case was technically unsatisfactory. Rearrangement of the FUS locus was not identified in 17 cases (1 case was technically unsatisfactory) (Table 1; Figure 2b).
Discussion
Angiomatoid fibrous histiocytoma is a mesenchymal neoplasm of intermediate malignancy that generally affects children and young adults. Initially described as angiomatoid ‘malignant’ fibrous histiocytoma,13 its behavior is more indolent than initially believed, although 2–11% recur locally, and rare (<1%) metastases have been observed.14 Usually involving the deep dermis/subcutis, it occurs most commonly within the extremities, followed by the trunk as well as the head and neck. Rare cases involve bone.8 The clinical features of the angiomatoid fibrous histiocytoma in our series (Table 1, summarized above) fit well within the age and anatomical distribution that has been described previously for this entity.1, 2, 3, 13, 14
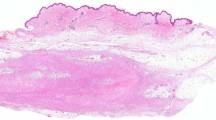
Classically, the neoplasm has a distinct low power appearance characterized by the presence of a fibrous pseudocapsule and a pericapsular lymphoplasmacytic infiltrate at the periphery of the neoplasm, which can simulate the appearance of a lymph node. More centrally, there is a multinodular proliferation of the neoplastic cells, which can be associated with blood-filled cystic spaces (‘pseudoangiomatoid’ spaces). The histogenesis/line of differentiation of the neoplastic cells is still debated; at higher power, the neoplastic cells are usually plump spindled to epithelioid cells in their cytomorphology, with ovoid vesicular nuclei, with a variably histiocytoid-to-myoid appearance. The neoplastic cells are usually bland and monomorphic, although examples with a greater degree of nuclear pleomorphism have been described.
Immunohistochemically, angiomatoid fibrous histiocytoma lacks a characteristic immunophenotype. In our series, at least focal desmin staining was found in almost every case. However, desmin was strongly positive (not focal) in only 69% of the cases in our study. Immunoreactivity for CD68 and CD99, two notoriously nonspecific stains, were noted in 67 and 80% of cases, respectively. It is noteworthy that three cases in our series were characterized by membranous staining for CD99, representing a potential pitfall in the diagnosis of Ewing's sarcoma/PNET, given that the majority of angiomatoid fibrous histiocytoma harbor rearrangements of EWSR1. EMA and SMA were helpful in differential diagnosis when positive; however, this occurred only 50 and 38% of the time, respectively. These results are similar to those previously reported in the literature.1, 2, 3, 15, 16
When the classic histological features are present, the diagnosis of angiomatoid fibrous histiocytoma is relatively straightforward. However, the morphological features described above are not always present. This problem is compounded by the lack of a characteristic immunohistochemical marker. Therefore, an ancillary molecular diagnostic test would be extremely helpful in difficult/atypical cases.
Our understanding of the molecular genetics of angiomatoid fibrous histiocytoma has increased substantially over the past decade. In early reports, two angiomatoid fibrous histiocytomas were found to harbor a FUS/ATF1 gene fusion, characterized by t(12;16)(q13;p11).4, 5 Subsequently, four additional angiomatoid fibrous histiocytomas were found to contain a t(12;22)(q13;q12) resulting in a EWSR1/ATF1 fusion.6, 7, 8 The finding that EWSR1 (22q12) can be substituted in the place of FUS (16p11) is not surprising as EWSR1 and FUS are both members of the TET family of RNA-binding proteins; indeed, EWSR1 has been found to replace FUS as the partner of CHOP (DDIT3)(12q13) in the gene fusions of myxoid/round cell liposarcomas.17, 18
Terra et al, found by screening with FISH probes for ATF1, that 4 of 14 cases (29%) harbored rearrangements of this gene. Of these four cases, three had the EWSR1/ATF1 fusion, whereas the remaining case displayed the FUS/ATF1 fusion. Significantly, ∼70% of the cases lacked a rearrangement of ATF1, suggesting that another genetic mechanism was involved in the majority of cases of angiomatoid fibrous histiocytomas.9
In a larger series, Antonescu et al found eight of nine angiomatoid fibrous histiocytoma to harbor a newly described EWS/CREB1 fusion resulting from a t(2;22)(q33;q12) in eight of nine angiomatoid fibrous histiocytoma, whereas the remaining case had the previously described EWSR1/ATF1 fusion. CREB1 and ATF1 are highly homologous genes; both are members of the cyclic adenosine monophosphate response element-binding (CREB) family of transcription factors.10 In the largest series to date, Rossi et al11 found that 13 of 14 cases to show the EWSR1/CREB1 fusion gene, whereas 1 case contained the EWSR1/ATF1 fusion gene.
From the literature (Table 2), ∼72% of angiomatoid fibrous histiocytomas studied at the molecular level harbor a EWSR1/CREB1 fusion. Another 21% contain a translocation involving EWSR1/ATF1, whereas the remaining 7% have a FUS/ATF1 fusion gene. Thus, ∼93% of angiomatoid fibrous histiocytoma have a rearrangement of EWSR1 (as manifested by the EWSR1/CREB1 and EWSR1/ATF1 fusion genes), whereas ∼7% of cases have a rearrangement of FUS.
Although the translocation partner was not identified in our study, our findings showed that EWSR1 is rearranged in 76% of angiomatoid fibrous histiocytoma, in keeping with the above-mentioned data. No rearrangements of FUS were identified, confirming that this gene is involved in only a small proportion of angiomatoid fibrous histiocytoma. Interestingly, ∼24% of cases lacked a rearrangement of either EWSR1 or FUS. One possibility is that some of the translocation-negative cases harbored breakpoints not detectable by the EWSR1 or FUS probes used in the study. Alternatively, a proportion of these translocation-negative cases could have a yet uncharacterized translocation/gene fusion, which does not involve rearrangement of EWSR1 or FUS. Additional studies are necessary to clarify this finding.
Immunoreactivity for CD99 was found in 7 of 16 (44%) cases in our study; a membranous staining pattern could be found at least focally in essentially all of the positive cases. Given that 76–93% of angiomatoid fibrous histiocytoma also harbor rearrangements of EWSR1, this represents a potential pitfall in the misdiagnosis of Ewing's sarcoma/PNET (which typically contains a membranous pattern of staining for CD99 and rearrangement of EWSR1). Recognition of this potential pitfall and careful attention to the cytological features of the tumor cells are essential to avoid overtreatment and/or unnecessary chemoradiation.
In summary, our findings show that FISH is an effective adjunct in the diagnosis of angiomatoid fibrous histiocytoma and affirm previous findings showing that EWSR1/CREB1 and EWSR1/ATF1 are the most common gene fusions/translocations found in angiomatoid fibrous histiocytoma. Although the translocation partner was not identified using this technique, FISH confers the advantage of detecting both the EWSR1/CREB1 and EWSR1/ATF1 gene fusions with a single molecular test. In addition, this technique can be carried out retrospectively on formalin-fixed, paraffin-embedded tissue and may be helpful in biopsies when histological features such as the characteristic lymphoid cuff are not present.
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
Weiss SW, Goldblum JR . Fibrohistiocytic tumors of intermediate malignancy. In: Weiss SW, Goldblum JR (eds) Enzinger and Weiss's Soft Tissue Tumors. 5th edn. Elsevier: Philadelphia, 2008. pp 390–394.
Fletcher CDM . Soft tissue tumors In: Fletcher CDM (ed). Diagnostic Histopathology of Tumors. Vol 2. 3rd edn. Elsevier Ltd: Philadelphia, 2007, pp 1574–1575.
Fletcher CDM, Unni KK, Merten F . World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, 2002, pp 194–195.
Waters BL, Panagopoulos I, Allen EF . Genetic characterization of angiomatoid fibrous histiocytoma identifies fusion of the FUS and ATF-1 genes induced by a chromosomal translocation involving bands 12q13 and 16p11. Cancer Genet Cytogenet 2000;121:109–116.
Raddaoui E, Donner LR, Panagopoulos I . Fusion of the FUS and ATF1 genes in a large, deep-seated angiomatoid fibrous histiocytoma. Diagn Mol Pathol 2002;11:157–162.
Hallor KH, Mertens F, Jin Y, et al. Fusion of the EWSR1 and ATF1 genes without expression of the MITF-M transcript in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 2005;44:97–102.
Somers GR, Viero S, Nathan PC, et al. Association of the t(12;22)(q13;q12) EWS/ATF1 rearrangement with polyphenotypic round cell sarcoma of bone: a case report. Am J Surg Pathol 2005;29:1673–1679.
Hallor KH, Micci F, Meis-Kindblom JM, et al. Fusion genes in angiomatoid fibrous histiocytoma. Cancer Lett 2007;251:158–163.
Terra SBSP, Folpe AL, Weiss SW, et al. Frequency and characterization of ATF1-fusion genes in angiomatoid fibrous histiocytoma. Mod Pathol 2007;21A, (Abstract 76).
Antonescu CR, Dal Cin P, Nafa K, et al. EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 2007;46:1051–1060.
Rossi S, Szuhai K, Ijszenga M, et al. EWSR1-CREB1 and EWSR1-ATF1 fusion genes in angiomatoid fibrous histiocytoma. Clin Cancer Res 2007;13:7322–7328.
Downs-Kelly E, Goldblum JR, Patel RM, et al. The utility of fluorescence in situ hybridization (FISH) in the diagnosis of myxoid soft tissue neoplasms. Am J Surg Pathol 2008;32:8–13.
Enzinger FM . Angiomatoid malignant fibrous histiocytoma: a distinct fibrohistiocytic tumor of children and young adults simulating a vascular neoplasm. Cancer 1979;44:2147–2157.
Costa MJ, Weiss SW . Angiomatoid malignant fibrous histiocytoma. A follow-up study of 108 cases with evaluation of possible histologic predictors of outcome. Am J Surg Pathol 1990;14:1126–1132.
Smith ME, Costa MJ, Weiss SW . Evaluation of CD68 and other histiocytic antigens in angiomatoid malignant fibrous histiocytoma. Am J Surg Pathol 1991;15:757–763.
Fletcher CD . Angiomatoid ‘malignant fibrous histiocytoma’: an immunohistochemical study indicative of myoid differentiation. Hum Pathol 1991;22:563–568.
Hosaka T, Nakashima Y, Kusuzaki K, et al. A novel type of EWS-CHOP fusion gene in two cases of myxoid liposarcoma. J Mol Diagn 2002;4:164–171.
Huang HY, Antonescu CR . Molecular variability of TLS-CHOP structure shows no significant impact on the level of adipogenesis: a comparative ultrastructural and RT-PCR analysis of 14 cases of myxoid/round cell liposarcomas. Ultrastruct Pathol 2003;27:217–226.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanas, M., Rubin, B., Montgomery, E. et al. Utility of FISH in the diagnosis of angiomatoid fibrous histiocytoma: a series of 18 cases. Mod Pathol 23, 93–97 (2010). https://doi.org/10.1038/modpathol.2009.138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.138
Keywords
This article is cited by
-
Angiomatoid fibrous histiocytoma: a series of seven cases including genetically confirmed aggressive cases and a literature review
BMC Musculoskeletal Disorders (2017)
-
Angiomatoid fibrous histiocytoma: novel MR imaging findings
Skeletal Radiology (2016)
-
Imaging of childhood angiomatoid fibrous histiocytoma with pathological correlation
Pediatric Radiology (2015)