Abstract
Urothelial dysplasia and carcinoma in situ (CIS) are related to recurrence and progression of urothelial carcinoma. Distinguishing CIS and dysplasia from reactive atypia is often difficult on the basis of histological features alone. Cytokeratin 20 (CK20), p53, and Ki-67 are related either to neoplastic change or prognosis in urothelial proliferations. The objective of the present study was to establish the immunohistochemical pattern of these three antibodies in urothelial dysplasia and CIS. Three groups of patients were evaluated: 40 nonneoplastic urothelial samples, 50 cases with histologically incontrovertible CIS, and 30 samples with nonconclusive atypical changes (atypia of unknown significance). Monoclonal antibodies (MoAb) against CK20, p53, and Ki-67 (MIB-1) were used on paraffin-embedded samples. Nonneoplastic urothelium showed no reactivity to CK20 except for umbrella cells; p53 and Ki-67 were negative or weakly positive in <10% of basal cells. In the CIS group, 42% showed positivity for all three MoAb; 44%, for two; and 14%, only for one. CK20 was positive through the full thickness of the urothelium in 72% of cases, p53 was positive in 80% of cases, and Ki-67, in 94% of cases. In the third group, the suspected dysplastic cells showed strong positivity in scattered cells through the epithelium in 75% of cases. Aberrant CK20 expression in urothelial cells plus overexpression of p53 and Ki-67 are indicators of dysplastic change in urothelial mucosa. Thus, immunohistochemistry is a useful tool to confirm the diagnosis of CIS and could be helpful to distinguish dysplastic changes from reactive atypia.
Similar content being viewed by others
INTRODUCTION
Dysplastic changes and particularly the presence of CIS in urothelium have important clinical implication both in prognosis and treatment of patients with urothelial neoplasms (1, 2, 3). Thus, identification of such cellular alteration is of great importance for correct management of these patients. Nevertheless, the interpretation of dysplastic change in urothelial cells is sometimes difficult on the basis of histological criteria alone, and frequently pathologists feel uncomfortable evaluating them, especially in small-cell CIS or when there is a significant loss of the urothelial lining with few atypical cells remaining. It is even more difficult to diagnose dysplasia in those cases in which urothelial atypia is observed in some cells but do not reach all the morphological criteria to be classified as CIS (1, 4, 5, 6).
Several biological markers have been reported in the literature to be good objective markers of progression of different neoplasms. The Ki-67 antigen recognized by the MIB-1 monoclonal antibody is a useful proliferation marker (7, 8, 9, 10). Overexpression of the p53 gene product has been reported to be a marker of progression in urothelial carcinoma (11, 12, 13, 14). CK20 is the last known component on the cytokeratin subgroup of intermediate filaments (15). Its expression has been described as an objective marker of the neoplastic change of urothelial cells (16, 17, 18, 19, 20, 21). All of these markers are easy to manage, and their interpretation has been quite standardized.
Thus, the purpose of the present study is to find whether the immunohistochemical expression of the panel CK20, p53, and Ki-67 in urothelium can be a tool for objectively distinguishing the cases with CIS and dysplastic urothelial changes from reactive nonneoplastic atypia.
MATERIALS AND METHODS
We studied 50 cases considered as incontrovertible urothelial CIS by two uropathologists after the WHO/ISUP criteria (1). Samples were collected from the files of the pathology department of our hospital. Samples from both ureter and urinary bladder were included. Forty cases of nonneoplastic urothelium, including samples of urinary bladder from necropsies and sections of ureter from nephrectomy specimens, were studied. We added a third group of 30 samples with cellular atypia that was suspicious but nonconclusive for dysplasia (atypia of unknown significance); these samples were obtained from mapping specimens in patients with concomitant urothelial carcinoma.
Immunohistochemistry was performed using the Envision (Dako Co, Carpinteria, California) method on 4-μm-thick sections from formalin-fixed, paraffin-embedded blocks. MoAb to Ki-67 (MIB-1; 1:200, DAKO, Carpinteria, CA), p53 (1:200, BioGenex, San Ramon, CA), and CK20 (1:200, DAKO) were used. Samples with known positive reactivity for each MoAb were used as positive controls. As a negative control, a section was processed in which the primary antibody was changed by PBS.
We recorded the percentage of positive cells for each marker in each group of cases as well as the distribution of expression and its intensity. As previously reported, Ki-67 was considered positive when >10% of cells showed nuclear positive expression (9); p53 was considered positive when ≥20% of cells showed nuclear positivity (12), and CK20 expression was considered aberrant when there was cytoplasmic expression on urothelial cells other than superficial umbrella cells (21).
RESULTS
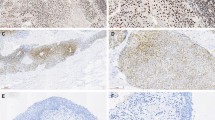
In nonneoplastic urothelium, CK20 was negative in the whole epithelium and expressed cytoplasmic positivity in a patchy way, just in umbrella cells in 14 cases. Ki-67 was expressed in basal cells with a weak-mild intensity in <5% of cells in 18 cases (45%). Similarly, p53 was expressed weakly in nuclei of some scattered cells, predominantly in a basal distribution, in 15 (37.5%) cases, always in <5% of cells. (Fig. 1)
When CIS immunohistochemical profile was analyzed, cases were considered positive by strict adherence to the criteria mentioned above. Thus, some cases were recorded as negative for some of the MoAb according to the percentage of positive cells, although some of them showed a variable number of intense positive cells (Cases 1, 30, 33, and 41). Results for the CIS group are summarized in Table 1. Among the 50 CIS cases, 21 (42%) showed reactivity for the whole panel, 22 (44%) reacted to two markers, and 7 (14%) reacted just to one of them. Ki-67 reacted in 47 cases (94%), showing >50% of positive cells in 18/47 cases (38.3%); p53 was considered positive in 40 cases (80%), with 28/40 (70%) showing >50% of positivity. CK20 showed positive reactivity in 36 cases (72%), with 31/36 (86%) showing a strong full-thickness positivity of >50% of atypical cells (Table 2, Fig. 2).
In the group of unknown significance atypia, we found 73.3% positivity for p53, 40% positivity for Ki-67, and 30% positivity for CK20. Also, 23.3% of cases were negative to the whole panel, 36.7% were positive just for one antibody, 13.3% were positive for two antibodies, and 26.7% were positive for the whole panel (Table 2). If we considered as positive the cases with intense, incontrovertible reaction to any of the MoAb in some cells, irrespective of the percentage, nuclear reactivity to p53 and Ki-67 was observed in 67.85% and 35.7% of cases, respectively. CK20 expression was cytoplasmic on scattered basal or intermediate cells in 25% of cases (Fig. 3). Differences between the two groups are reflected in Table 3.
DISCUSSION
Urothelial carcinoma is a recurrent neoplasm with a significant number of cases in which neoplasm progresses to an infiltrating, very aggressive disease. CIS is a neoplastic change of the urothelium considered to be a high-grade neoplasm and is an indicator of progression of urothelial neoplasm that requires specific treatment (1, 2, 22). There are cases in which cell pleomorphism is so evident that it is easy to make a diagnosis of CIS. Nevertheless, there are still many cases such as small-cell CIS, or the clinging form with denuded epithelium and few remaining cells, in which pathologists do not feel absolutely confident in the diagnosis of CIS. Urothelial dysplasia has been considered a putative precursor of CIS, an invasive urothelial carcinoma of the urinary tract (1). Nevertheless, the distinction between reactive and dysplastic changes has not been resolved, even after publication of the WHO/ISUP classifications (4, 5, 6). There still are no definite morphological criteria to diagnose CIS, and there is great inter- and intraobserver disagreement. Furthermore, little is known about the evolution of cases with dysplastic changes in the absence of CIS or invasive neoplasia (23). Finding objective markers to aid in the distinction of true dysplastic changes from reactive cellular alterations is one of the challenges for uropathologists. Proliferation markers, oncogene products, adhesion molecules, and many other types of biological markers (24, 25, 26) have been studied to find a way to distinguish those patients with a poorer prognosis of their disease in order to offer the best treatment. In this way, p53 uniformly has been reported as a marker of progression in transitional cell carcinoma (11, 12, 13, 14). CK20 has been proposed as a marker of neoplastic change as well as a predictor for progression of urothelial carcinoma (18, 19, 20, 21). Ki-67 is a proliferation marker, with demonstrated usefulness in many neoplasms in determining their progression (7, 8, 9, 10, 11).
Our results confirm the normal immunohistochemical profile in nonneoplastic urothelium. Harden et al. (21) have already described the aberrant pattern of expression for CK20 as the presence of cytoplasmic immunostaining of urothelial cells other than superficial, umbrella cells. Ki-67 and p53 in nonneoplastic urothelium have been considered to be nonexpressed. Some of our cases have shown some weak positivity in the basal cell layer. Because of that, we consider positivity for MoAb only when there is intense nuclear reaction.
According to our results, the immunohistochemical panel composed of p53, Ki-67, and CK20 is useful for confirming the presence of dysplastic changes in the urothelium. The positivity was always strong and, in many cases, in a high percentage of cells (ranging from 50–90%). This immunohistochemical profile is clearly different from the one observed in normal urothelium, in which the whole panel is always negative or just weakly positive in few cells. From this comparison, we can conclude that our proposed immunohistochemical panel for studying dysplastic urothelial changes is adequate. McKenney et al. (27) recently have published similar results for CK20 and p53 in a series of 21 CIS cases and 25 nonneoplastic urothelia. Although our results are in agreement with those of that study, we have found that Ki-67 is the most constant marker associated with CIS and in a higher percentage. In the same way, in the group of atypical nonconclusive changes, Ki-67 expression was the most evident and confident. Although in the control group there was some positivity for Ki-67, it was always weak and in a basal distribution.
In daily practice we have found the immunohistochemical panel with CK20, Ki-67, and p53 MoAb of great help in confirming CIS, particularly in cases with denuded epithelium but suspicious cells or in small-cell CIS.
Because the immunohistochemical profile obtained for the group of unknown-significance atypia was also substantially different from the normal, nonneoplastic urothelium, we could anticipate that the expression of those MoAb might be useful for distinguishing preneoplastic urothelial changes from reactive atypia. Among this group of cases, we found intense and aberrant expression for the MoAb evaluated. Evaluation of those markers can be of aid in better defining the histological criteria of urothelial dysplasia. According to our results, the presence of intense positivity of suspicious cells for at least one of the MoAb would confirm the presence of dysplastic changes. Prospective clinical correlation of large series with patients without concomitant CIS or invasive carcinoma is needed to confirm that hypothesis and to understand its clinical implication.
References
Epstein JI, Amin MB, Reuter VR, Mostofi FK . The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol 1998; 22(12): 1435–1448.
Cheng L, Cheville JC, Neumann RM, Bostwick DG . Natural history of urothelial dysplasia of the bladder. Am J Surg Pathol 1999; 23(4): 443–447.
Amling CL . Diagnosis and management of superficial bladder cancer. Curr Probl Cancer 2001; 5(4): 219–278.
Murphy WM, Busch C, Algaba F . Intraepithelial lesions of urinary bladder: morphologic considerations. Scand J Urol Nephrol Suppl 2000; 205: 67–81.
McKenney JK, Gomez JA, Desai S, Lee MW, Amin MB . Morphologic expression of urothelial carcinoma in situ: a detailed evaluation of its histologic patterns with emphasis on carcinoma in situ with microinvasion. Am J Surg Pathol 2001; 25(3): 356–362.
Milord RA, Lecksell K, Epstein JI . An objective morphologic parameter to aid the diagnosis of flat urothelial carcinoma in situ. Hum Pathol 2001; 32(9): 997–1002.
Gerdes J, Becker MHG, Keyt G, Cattoretti G . Immunohistochemical detection of tumor growth fraction (Ki-67 antigen) in formalin fixed and routinely processed tissue. J Pathol 1992; 168: 65–87.
Tsuji M, Kojima K, Murakami Y, Kanayama H, Kagawa S . Prognostic value of Ki-67 antigen and p53 protein in urinary bladder cancer: immunohistochemical analysis of radical cystectomy specimens. Br J Urol 1997; 79: 367–372.
Mazerolles C, Rishmann P, Chopin D . Usefulness of Mib-1 monoclonal antibody in assessing the proliferative index in human bladder carcinoma: comparison with Ki-67 antibody. Histopathology 1994; 25: 563–568.
Fontana D, Bellina M, Gubetta L . Monoclonal antibody Ki-67 in the study of proliferative activity of bladder carcinoma. J Urol 1992; 148: 1149–1151.
Cina SJ, Lancaster-Weis KJ, Lecksell K, Epstein JI . Correlation of Ki-67 and p53 with the New World Health Organization/International Society of urological pathology classification system for urothelial neoplasia. Arch Pathol Lab Med 2001; 125: 646–651.
Cordon-Cardo C, Reuter V . Alterations of tumor suppressor genes in bladder cancer. Semin Diagn Pathol 1997; 14: 123–132.
Wright C, Mellon K, Johnston P . Expression of mutant p53, c-erb-B2 and epidermal growth factor receptor in transitional cell carcinoma of the human urinary bladder. Br J Cancer 1991; 63: 967–970.
Fujimoto K, Yamada Y, Okajima E . Frequent association of p53 gene mutation in invasive bladder cancer. Cancer Res 1992; 52: 1393–1398.
Moll R, Löwe A, Laufer J, Franke WW . Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol 1992; 140(2): 427–447.
Chu P, Wu E, Weiss LM . Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol 2000; 13(9): 962–972.
Desai S, Lim SD, Jimenez RE, et al. Relation of cytokeratin 20 and CD44 protein expression with WHO/ISUP grade pTa and pT1 papillary urothelial neoplasia. Mod Pathol 2000; 13(12): 1315–1323.
Harnden P, Eardly I, Joyce AD, Southgate J . Cytokeratin 20 as an objective marker of urothelial dysplasia. Br J Urol 1996; 78: 870–875.
Klein A, Zemer R, Buchumensky V, Klaper R, Nisserkon I . Expression of cytokeratin 20 in urinary cytology of patients with bladder carcinoma. Cancer 1998; 82: 349–354.
Alsheik A, Mohamedali Z, Jones E, Masterson J, Gilks CB . Comparison of the WHO/ISUP classification and cytokeratin expression in predicting the behaviour of low-grade papillary urothelial tumors. Mod Pathol 2001; 14(4): 267–272.
Harnden P, Mahmood N, Southgate J . Expression of cytokeratin 20 redefines urothelial papillomas of the bladder. Lancet 1999; 353: 974–977.
Cheng L, Cheville JC, Neumann RM . Survival of patients with carcinoma in situ of the urinary bladder. Cancer 1999; 85: 2469–2474.
Sarkis AS, Dalbagni G, Cordon-Cardo C, et al. Association of p53 nuclear overexpression and tumor progression in carcinoma in situ of the bladder. J Urol 1994; 152: 388–392.
Romero J, Alos L, Mallofre C, Solé M, Gutierrez R, Alcover J, et al. Bladder wash cytology and flow cytometry for the diagnosis of transitional cell carcinoma of the urinary bladder. Eur Urol 1992; 21: 13–15.
Zaher A, Sheridan T . Tumor markers in the detection of recurrent transitional cell carcinoma of the bladder. Acta Cytol 2001; 45: 575–581.
Cassel A, Rahat MA, Lahat N, Lindenfeld N, Mecz Y, Stein A . Telomerase activity and cytokeratin 20 as markers for the detection and follow-up of transitional cell carcinoma: an unfulfilled promise. J Urol 2001; 166: 841–844.
McKenney JK, Desai S, Cohen C, Amin MB . Discriminatory immunohistochemical staining of urothelial carcinoma in situ and nonneoplastic urothelium. Am J Surg Pathol 2001; 25: 1074–1078.
Acknowledgements
The authors acknowledge Mrs. Montserrat Sanchez for her accurate technical support.
Supported in part by Grant SAF 99/020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallofré, C., Castillo, M., Morente, V. et al. Immunohistochemical Expression of CK20, p53, and Ki-67 as Objective Markers of Urothelial Dysplasia. Mod Pathol 16, 187–191 (2003). https://doi.org/10.1097/01.MP.0000056628.38714.5D
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000056628.38714.5D
Keywords
This article is cited by
-
Does the addition of AMACR to CK20 help to diagnose challenging cases of urothelial carcinoma in situ?
Diagnostic Pathology (2019)
-
Histopathologic and molecular comparative analyses of intravesical Aurora kinase-A inhibitor Alisertib with bacillus Calmette–Guérin on precancerous lesions of bladder in a rat model
International Urology and Nephrology (2018)