Abstract
There is controversy over the histogenesis of Kaposi's sarcoma (KS) from lymphatic or blood vessel endothelium. D2-40 is a novel monoclonal antibody to an Mr 40,000 O-linked sialoglycoprotein that reacts with a fixation-resistant epitope on lymphatic endothelium. We sought to establish the selectivity of D2-40 for lymphatic endothelium in normal tissues and compare its reactivity with the expression of the widely used vascular endothelial marker CD31 in a series of 62 formalin-fixed and paraffin-embedded vascular lesions including KS. In normal tissues, D2-40 stained the endothelium of lymphatic channels but not of blood vessels, including arteries and capillaries defined by reactivity with the blood vessel endothelial marker PAL-E. In our series of vascular lesions, D2-40 stained lymphangiomas (10/10), benign tumors of undisputed lymphatic origin, but not benign neoplasms or tumorlike lesions of blood vessel origin, including hemangiomas (0/10), glomus tumors (0/3), angiolipomas (0/2), pyogenic granulomas (0/2), vascular malformations (0/2), hemangiopericytoma (0/1), or hemangioendothelioma (0/1). D2-40 stained all cases of cutaneous KS (24/24) at all stages of progression, including patch, plaque, and nodular stages, supporting the concept that this disease originates from a cell type capable of undergoing lymphatic differentiation. D2-40 also stained three of seven angiosarcomas, indicating that a subset of these tumors can undergo at least partial differentiation along the lymphatic endothelial lineage and could be classified as lymphangiosarcomas. In comparison, CD31 was expressed in all benign and malignant vascular lesions, except for glomus tumors (0/3) and 5/10 lymphangiomas, in which staining was absent. We conclude that D2-40 is a new selective marker of lymphatic endothelium in normal tissues and vascular lesions and is valuable for studying benign and malignant vascular disorders in routinely processed tissue specimens.
Similar content being viewed by others
INTRODUCTION
Kaposi's sarcoma (KS) is a vascular proliferative disorder with four epidemiological forms: classic, African endemic, iatrogenic transplant related, and epidemic acute immune deficiency syndrome (AIDS) related (1). KS is usually limited to the skin but may involve mucus membranes, visceral organs, and lymph nodes in the more aggressive forms (2). For cutaneous KS, a histopathological progression from patch to plaque and nodular stages correlates with a clinical progression from macules to palpable tumors (2). The patch stage is characterized by networks of thin-walled irregular endothelial-lined spaces; the plaque stage, by sparse spindle cells intermingled among irregular endothelial-lined spaces; and the nodular stage, by sheets or fascicles of spindle cells (2).
Although the exact pathogenesis of KS is not defined, it has been suggested that a chronic cytokine-mediated proliferative state of an endothelial cell, possibly triggered by activation of a latent KS-associated herpes virus (KSHV) infection, promotes transformation to a neoplastic cell (1, 3, 4). The origin of KS from blood vessel or lymphatic endothelium is controversial. Although some early studies failed to detect the presence of blood vessel endothelial markers in KS (5), other studies reported the presence of traditional blood vessel endothelial markers including CD31, CD34, and von Willebrand factor (vWF, Factor VIII–related antigen) (6, 7, 8, 9). The latter findings are compatible with a theory of origin of KS from blood vessel endothelium. However, this interpretation is complicated by recent observations that the above blood vessel endothelial markers are also weakly expressed on lymphatic endothelium (9, 10, 11). Furthermore, ultrastructural features and the absence of the blood vessel endothelium–specific PAL-E antigen (12) from KS argue against an origin from blood vessel endothelial cells (13, 14, 15). These considerations have led some authors to suggest that KS originates from lymphatic endothelium (5, 14). This notion has been reinforced recently by the demonstration of a uniform expression of two lymphatic endothelial markers, vascular endothelial growth factor receptor-3 (VEGFR-3; 16) and podoplanin (17), in KS (18, 19, 20). However, VEGFR-3 has also been shown to be expressed by neoplastic blood vessel endothelia in both benign and malignant vascular tumors and, in this situation, loses its selectivity for lymphatic endothelium (21). Therefore, we wished to reexamine these results with an independent marker of lymphatic endothelium.
We recently observed that D2-40, a monoclonal antibody (mAb) to an Mr 40,000 O-linked sialoglycoprotein (22), was a selective marker of lymphatic endothelium. In this study, we examined the expression of this novel marker of lymphatic endothelium in normal tissues and compared its reactivity with the expression of CD31 in a series of 62 vascular lesions, including 24 cases of cutaneous KS at different stages of progression.
MATERIALS AND METHODS
Formalin-fixed, paraffin-embedded blocks of normal tissue, 62 vascular lesions (24 cutaneous KS, 7 angiosarcomas, 10 lymphangiomas, 10 hemangiomas, 3 glomus tumors, 2 angiolipomas, 2 pyogenic granulomas, 2 vascular malformations, 1 hemangiopericytoma, 1 hemangioendothelioma), 28 nonvascular soft-tissue tumors (10 leiomyosarcomas, 6 neurofibromas, 6 dermatofibromas, 4 dermatofibrosarcoma protuberans, 2 malignant fibrous histiocytomas), as well as frozen samples of tonsil, were obtained from the Department of Pathology, Sunnybrook and Women's College Health Sciences Center, University of Toronto, Ontario, Canada. The KS specimens included patch, plaque, and nodular lesions from patients with classic and AIDS- and transplant-related KS. None of the angiosarcomas were associated with lymphedema or preexisting lymphangioma.
MAb D2-40 (IgG1) was purified from mouse ascitic fluid, as previously described (22), and stored at a concentration of 0.7 mg/mL under sterile conditions at 4° C. The mAb to an undefined blood vessel endothelial antigen, clone PAL-E, (hybridoma supernatant 15 μg/mL from Monosan), whose reactivity is restricted to frozen sections of tissues, was purchased through Cederlane Laboratories (Hornby, Ontario, Canada). The mAb to CD31 (Clone JC/70A, hybridoma supernatant) was purchased from DAKO (Carpinteria, CA).
Sections of paraffin-embedded tissues (5 μm) were dewaxed through graded concentrations of ethanol. For immunostaining, heat-inducted epitope retrieval by microwave pretreatment (800 W for 10 min) was only performed for CD31. The sections were first incubated in methanol containing 3% H2O2 to inactivate endogenous peroxidase. The sections were then incubated with mAb D2-40 (0.1 μg/mL), or anti-CD31 (1:40 dilution), followed sequentially by biotinylated goat anti-mouse immunoglobulin (IgG) antibody (Zymed, San Francisco, CA) at a 1:200 dilution and a horseradish peroxidase–avidin conjugate (DAKO) at a 1:500 dilution. For color development, the sections were incubated with 3,3′-diamino-benzidine. Adjacent cryostat sections (5 μm) of quick-frozen tissues were air-dried and fixed in cold acetone for 10 min. After inactivation of the endogenous peroxidase as described above, the sections were immunostained with mAb PAL-E (0.15 μg/mL) or D2-40 (0.1 μg/mL), also as described above.
RESULTS
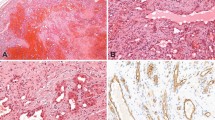
Consecutive cryostat sections of human tonsil were stained with mAb D2-40 or PAL-E (that is specific for endothelial cells of blood vessels; 12). These antibodies stained nonoverlapping populations of thin-walled endothelial channels in adjacent sections. PAL-E stained blood vessels (Fig. 1A, arrowheads) that did not stain with D2-40 (Fig. 1B). Conversely, D2-40 stained lymphatic channels (Fig. 1B, arrowheads) that did not stain with PAL-E (Fig. 1A). In paraffin sections of normal breast, D2-40 stained the endothelium of lymphatic channels (Fig. 1C, arrowheads), but not the endothelium of an artery in a vascular bundle (Fig. 1C, arrow).
Selective staining of lymphatic vessels with D2-40 in normal tissues. A and B, consecutive frozen sections of tonsil were stained with PAL-E (A) or D2-40 (B), showing positive endothelial staining of distinct populations of vessels (arrowheads). C and D, paraffin sections of breast were stained with D2-40 showing positive staining of lymphatics (arrowheads) and absence of staining of artery (C) or capillary (D; arrows). Note the presence of lymphocytes in the lumen of the lymphatic vessel in (D). (Original magnification, A and B: 100 ×; C: 300 ×; D: 400 ×).
D2-40 also stained the endothelium of a lymphatic channel containing lymphocytes (Fig. 1D, arrowhead), but not the adjacent capillary (Fig. 1D, arrow). The results of immunostaining of a series of vascular lesions with mAb D2-40 and for CD31 are summarized in Table 1. D2-40 reacted strongly with all cases of cutaneous KS (24/24) at the patch (Fig. 2B–C), plaque, and nodular (Fig. 2E–F) stages of progression. D2-40 also reacted with the endothelium lining the sinusoidal spaces in all lymphangiomas (10/10; Fig. 3A) but did not react with any hemangiomas (0/10), glomus tumors (0/3), angiolipomas (0/2), pyogenic granulomas (0/2), vascular malformations (0/2), hemangiopericytoma (0/1), or hemangioendothelioma (0/1). Among angiosarcomas, there was diffuse immunostaining of the neoplastic endothelial cells in 3/7 cases (Fig. 3C) and absence of staining in 4/7 cases (Fig. 3B). Of the three D2-40-positive angisarcomas, all exhibited hobnail endothelial cells, and one had, in addition, interspersed lymphoid aggregates. None of these features were seen in D2-40-negative angiosarcomas. With respect to nonvascular soft-tissue tumors, D2-40 did not stain neurofibromas (0/6) or dermatofibrosarcoma protuberans (0/4) and reacted with a fraction of leiomyosarcomas (3/10), dermatofibromas (2/6), and malignant fibrous histiocytomas (1/2). CD31 was positive in all cases of KS, hemangioma, angiolipoma, pyogenic granuloma, vascular malformation, hemangiopericytoma, hemangioendothelioma, and angiosarcoma (Fig. 3E–F). CD31 did not stain glomus tumors (0/3). Among lymphangiomas, there was absence of staining for CD31 in 5/10 cases (Fig. 3D) and weak staining in 5/10 cases.
Staining of Kaposi's sarcoma with D2-40. A–C, patch-stage Kaposi's sarcoma stained with hematoxylin and eosin (A) or with D2-40 (B and C). There is diffuse positive staining of the irregular endothelial lined spaces with D2-40 in B and C. D–F, nodular-stage Kaposi's sarcoma stained with hematoxylin and eosin (D) or D2-40 (E and F). There is diffuse positive staining of the spindle cells with D2-40 in E and F (original magnification, A, B, D, and E: 100 ×; C and F, 300 ×).
Staining of lymphangioma and angiosarcoma with D2-40 or anti-CD31. AandD, consecutive paraffin sections of a lymphangioma were stained with D2-40 (A), showing strong positive staining of sinusoidal spaces, or with anti-CD31 (D), showing absence of staining. BandE, consecutive paraffin sections of an angiosarcoma were stained with D2-40 (B), showing absence of staining, or with anti-CD31 (E), showing diffuse positive staining. Cand F, consecutive paraffin sections of an angiosarcoma were stained with D2-40 (C), showing diffuse positive staining, or with anti-CD31 (F), likewise showing diffuse positive staining. Note absence of staining of the endothelial lining of a normal artery with D2-40 in C (arrow) and positive staining with anti-CD31 in F (arrowhead; original magnification, A to F: 100 ×).
DISCUSSION
D2-40 was previously reported to react with an oncofetal antigen expressed in fetal testis and on the surface of testicular germ cell tumors, but not in adult testis (22). In this study, we report, for the first time, that this mAb is also a new selective marker of lymphatic endothelium and does not react with blood vessel endothelium, as demonstrated by the following results.
First, D2-40 reacted with the endothelium of lymphatic channels presumptively characterized by their morphological appearance in normal tissues. Specifically, these were flattened or open spaces lined by a single layer of endothelial cells whose lumen was sometimes filled with lymphocytes. The endothelium of blood vessels including arteries, veins, or capillaries did not stain with D2-40. To definitively distinguish thin-walled lymphatics from capillaries and venules, we compared the immunostaining of adjacent frozen sections of tonsil with mAb D2-40 and PAL-E, a recognized specific marker of blood vessel endothelium whose reactivity is limited to frozen sections of tissues (12). In these sections, D2-40 and PAL-E stained nonoverlapping populations of vessels, confirming the selective reactivity of D2-40 with lymphatic channels.
D2-40 reacted effectively with a fixation-resistant epitope on lymphatic endothelial cells in conventionally processed formalin-fixed and paraffin-embedded tissues without the requirement for epitope retrieval. This facilitated our use of this reagent to survey a series of vascular tumors and tumor-like lesions from our archival collection. Initially, we demonstrated that the selectivity of D2-40 for lymphatic endothelium in normal tissues was maintained for the neoplastic and reactive endothelium of benign vascular lesions. In particular, D2-40 reacted with the endothelial cells lining the sinusoidal spaces of lymphangiomas, (10/10, 100% sensitivity), but not the endothelial cells of hemangiomas (0/10, 100% specificity) or other vascular lesions of blood vessel origin, including glomus tumors (0/3), angiolipomas (0/2), pyogenic granulomas (0/2), vascular malformations (0/2), hemangiopericytoma (0/1), or hemangioendothelioma (0/1, 100% specificity). The endothelial antigen, CD31, which is expressed uniformly on the endothelium of blood vessels but only focally on lymphatics (10, 11), was present in all vascular lesions examined, with the exception of glomus tumors (0/3) and lymphangiomas, where it was not detected on the endothelium lining the sinusoidal spaces in 50% (5/10) of the cases. After establishing the selectivity of D2-40 for lymphatic endothelium in both normal tissues and benign vascular tumors and tumor-like lesions, we surveyed a series of KS and angiosarcomas for reactivity with this antibody.
All cases of cutaneous KS at different stages of progression reacted strongly with D2-40 (24/24, 100% sensitivity). D2-40 stained 100% of the endothelial cells lining the irregular spaces in patch- and plaque-stage lesions and the spindle cells in the plaque- and nodular-stage tumors. All cases of KS were also weakly positive for CD31 expression. These results suggest that KS originates from lymphatic endothelium, or, at the very least, from a pluripotent cell type capable of differentiating along both the lymphatic and blood vessel endothelial lineages, in agreement with similar conclusions reached on the basis of expression of VEGFR-3 and podoplanin (18, 19, 20). In the case of VEGFR-3, the interpretation of the results is complicated by the fact that this marker is selective for lymphatic endothelium in normal tissues, but not in vascular tumors, where it reacts with the neoplastic endothelium of benign and malignant blood vesslels (21).
Interestingly, our results with angiosarcomas indicate that a substantial subset (3/7) of these malignant vascular tumors can initiate a differentiation program along the lymphatic endothelial lineage based on their immunoreactivity with D2-40, again in agreement with similar conclusions based on the expression of the lymphatic endothelial markers VEGFR-3, podoplanin, and the β-chemokine receptor D6 (17, 20, 23). In the sample of angiosarcomas surveyed in our study, there was a clear distinction between the tumors that showed absence of staining with D2-40 and those that showed diffuse staining (100% of tumor cells positive) with the antibody. All the angiosarcomas examined in our study stained strongly for CD31 expression. Only the D2-40–positive angiosarcomas exhibited hobnail endothelial cells, as previously reported for VEGFR-3– positive angiosarcomas (20). Our results of D2-40 and CD31 staining suggest that angiosarcomas can be divided into subsets that originate from one of two distinct progenitor cell types, one restricted to differentiate along the blood vessel endothelial lineage and the other capable of differentiating along both the lymphatic and blood vessel lineages. Thus, a panel of markers of lymphatic endothelium including D2-40 would be useful to distinguish these tumors.
Among vascular lesions that could be considered in the differential diagnosis of KS, D2-40 did not react with hemangiomas (0/10), vascular malformations (0/2), hemangiopericytoma (0/1), or hemangioendothelioma (0/1) and reacted with 3/7 angiosarcomas. Therefore, with the exception of those angiosarcomas that express D2-40, this antibody can be used practically to distinguish these lesions from KS. With respect to nonvascular soft-tissue tumors, D2-40 did not react with neurofibromas (0/6) or dermatofibrosarcoma protuberans (0/4) and reacted with a fraction of leiomyosarcomas (3/10), dermatofibromas (2/6), and malignant fibrous histiocytomas (1/2). This indicates that D2-40 is selective for lymphatic versus vascular endothelium in both normal tissues and vascular tumors and tumor-like lesions but is not absolutely specific for lymphatic endothelium because it also reacts with a subset of nonvascular soft-tissue tumors of mesenchymal origin.
In summary, we report that D2-40 is a new selective monoclonal marker of lymphatic endothelium and an excellent immunohistochemical marker of KS. D2-40 also stains a subset of angiosarcomas and can be used in a panel of markers to subtype these tumors on the basis of their capacity to initiate a program of differentiation along the lymphatic endothelial lineage.
References
Antman K, Chang Y . Kaposi's sarcoma. N Engl J Med 2000; 342: 1027–1038.
Tapparo JW, Conant MA, Wolfe SF, Berger TG . Kaposi's sarcoma. Epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol 1993; 28: 371–395.
Gallo RC . The enigmas of Kaposi's sarcoma. Science 1998; 282: 1837–1839.
Karasek MA . Origin of spindle-shaped cells in Kaposi's sarcoma. Lymphology 1994; 27: 41–44.
Beckstead JH, Wood GS, Fletcher V . Evidence for the origin of Kaposi's sarcoma from lymphatic endothelium. Am J Pathol 1985; 119: 294–300.
Facchetti F, Lucini L, Gavazzoni R, Callea F . Immunomorphological analysis of the role of blood vessel endothelium in the morphogenesis of cutaneous Kaposi's sarcoma: a study of 57 cases. Histopathology 1988; 12: 581–593.
Sankey EA, More L, Dhillon AP . QB Ind/10: a new immunostain for the routine diagnosis of Kaposi's sarcoma. J Pathol 1990; 161: 267–271.
Hoerl HD, Goldbloom JR . Immunoreactivity patterns of CD31 and CD68 in 28 cases of Kaposi's sarcoma: evidence supporting endothelial differentiation in the spindle cell compartment. Appl Immunohistochem 1997; 5: 173–178.
Miettinen M, Lindenmayer AE, Chaubal A . Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens—evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol 1994; 7: 82–90.
Erhard H, Rietveld FJR, Bröcker EB, de Waal RMW, Ruiter DJ . Phenotype of normal cutaneous microvasculature. J Invest Dermatol 1996; 106: 135–140.
Sauter B, Foedinger D, Sterniczky B, Wolff K, Rappersberger K . Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells: differential expression of CD31, CD34, and type IV collagen with lymphatic endothelial cells vs blood capillary endothelial cells in normal human skin, lymphangioma, and hemangioma in situ. J Histochem Cytochem 1998; 46: 165–176.
Schlingman RO, Dingjan GM, Emeis JJ, Blok J, Warnaar SO, Ruiter DJ . Monoclonal antibody PAL-E specific for endothelium. Lab Invest 1985; 52: 71–76.
Jones RR, Spaull JS, Spry C, Jones EW . Histogenesis of Kaposi's sarcoma in patients with and without acquired immune deficiency syndrome (AIDS). J Clin Pathol 1986; 39: 742–749.
Rappersberger K, Tschachler E, Zonzits E, Gillitzer R, Hatzakis A, Kaloterakis A, et al. Endemic Kaposi's sarcoma in human immunodeficiency virus type 1-seronegative persons: demonstration of retrovirus-like particles in cutaneous lesions. J Invest Dermatol 1990; 95: 371–381.
Stürzl M, Brandstetter H, Roth KW . Kaposi's sarcoma: a review of gene expression and ultrastructure of KS spindle cells in vivo. AIDS Res Hum Retroviruses 1992; 8: 1753–1762.
Lymboussaki A, Partanen TA, Olofsson B, Thomas-Crussels J, Fletcher CDM, de Waal RMW, et al. Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol 1998; 153: 395–403.
Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries. Podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999; 154: 385–394.
Jussila L, Valtola R, Partanen TA, Salven P, Heikkilä P, Matikainen M-T, et al. Lymphatic endothelium and Kaposi's sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res 1998; 58: 1599–1604.
Weninger W, Partanen TA, Breiteneder-Geleff S, Mayer C, Kowalski H, Mildner M, et al. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi's sarcoma tumor cells. Lab Invest 1999; 79: 243–251.
Folpe AL, Veikkola T, Valtola R, Weiss SW . Vascular endothelial growth factor receptor-3 (VEGFR-3): a marker of vascular tumors with presumed lymphatic differentiation, including Kaposi's sarcoma, Kaposiform and Dabska-type hemangioendotheliomas, and a subset of angiosarcomas. Mod Pathol 2000; 13: 180–185.
Partanen TA, Alialo K, Miettinen M . Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor-3 in 185 vascular tumors. Cancer 1999; 86: 2406–2411.
Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, et al. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumors. Br J Cancer 1999; 80: 569–578.
Nibbs RJB, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JDM, et al. The β-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol 2001; 158: 867–877.
Acknowledgements
We wish to thank Mr. Kevin Kwok for excellent technical assistance. This work was supported in part by a grant to AM from the National Sciences and Engineering Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kahn, H., Bailey, D. & Marks, A. Monoclonal Antibody D2-40, a New Marker of Lymphatic Endothelium, Reacts with Kaposi's Sarcoma and a Subset of Angiosarcomas. Mod Pathol 15, 434–440 (2002). https://doi.org/10.1038/modpathol.3880543
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880543
Keywords
This article is cited by
-
Development of a new histological identification method of human sinoatrial node suitable for immunohistochemical study
Anatomical Science International (2023)
-
Epigenetic control in Kaposi sarcoma-associated herpesvirus infection and associated disease
Seminars in Immunopathology (2020)
-
Kaposi sarcoma
Nature Reviews Disease Primers (2019)
-
Primary Angiosarcoma Pancreas: a Case Report of an Exceptional Localization
Journal of Gastrointestinal Cancer (2019)
-
The glomus tumor resorbed bone and teeth in the mandible: a case report
Head & Face Medicine (2018)