Abstract
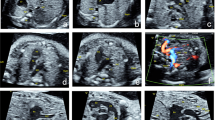
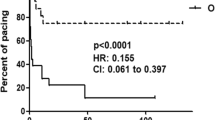
Fetuses with complete heart block have an increased mortality with most deaths occurring in utero or during infancy. The cardiac evaluation of these fetuses is difficult since the ventricular rate is low and the heart is dilated. We have implemented a strategy that includes the biophysical profile, which assesses fetal well-being, in combination with the cardiovascular profile that assesses cardiac function and the circulation. We present two cases of fetal complete heart block in which early delivery was recommended due to worsening cardiovascular profile scores. Biophysical profile scores were normal. Both babies were successfully treated, despite having risk factors that predicted poor outcomes. We hypothesize that our management protocol initiated intervention before fetal compromise, hydrops, and myocardial damage occurred. We recommend an evaluation of heart function in addition to an assessment of fetal well-being in fetuses with complete heart block. Early delivery should be considered if there is evidence of distress and/or deteriorating cardiac function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol 1998;31:1658–1666.
Eronen M, Siren MK, Ekblad H, Tikanoja T, Julkunen H, Paavilainen T . Short and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics 2000;106:86–91.
Groves AMM, Allan LD, Rosenthal E . Outcome of isolated congenital complete heart block diagnosed in utero. Heart 1996;75:190–194.
Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, Hornberger LK . Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. J Am Coll Cardiol 2002;39:130–137.
Michaelsson M, Engle MA . Congenital complete heart block: an international study of the natural history. Cardiovasc Clin 1972;4:85–101.
Manning FA, Platt LD, Sipos L . Antepartum fetal evaluation: development of a fetal biophysical profile score. Am J Obstet Gynecol 1980;136:787–795.
Vintzileos AM, Gaffney SE, Salinger LM, Campbell WA, Nockinson DJ . The relationship between fetal biophysical profile and cord pH in patients undergoing cesarean section before the onset of labor. Obstet Gynecol 1987;70:196–201.
Huhta JC . Right ventricular function in the human fetus. J Perinat Med 2001;29:381–389.
Moak JP, Barron KS, Hougen TJ, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol 2001;37:238–242.
Udink ten Cate FEA, Breur JMPJ, Cohen MI, et al. Dilated cardiomyopathy in isolated congenital complete atrioventricular block: early and long-term risk in children. J Am Coll Cardiol 2001;37:1129–1134.
Nield LE, Silverman ED, Taylor GP, et al. Maternal anti-ro and anti-la antibody-associated endocardial fibroelastosis. Circulation 2002;105:843–848.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Donofrio, M., Gullquist, S., Mehta, I. et al. Congenital Complete Heart Block: Fetal Management Protocol, Review of the Literature, and Report of the Smallest Successful Pacemaker Implantation. J Perinatol 24, 112–117 (2004). https://doi.org/10.1038/sj.jp.7211038
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211038
This article is cited by
-
Fetal arrhythmias: prenatal evaluation and intrauterine therapeutics
Italian Journal of Pediatrics (2020)
-
Fetal Arrhythmias: Genetic Background and Clinical Implications
Pediatric Cardiology (2019)
-
Prenatal Evaluation and Management of Fetuses Exposed to Anti-SSA/Ro Antibodies
Pediatric Cardiology (2012)
-
The cerebroplacental Doppler ratio predicts postnatal outcome in fetuses with congenital heart block
Journal of Perinatology (2008)