Abstract
Clay minerals, charcoal and metal oxides are essential parts of the soil matrix and strongly influence the formation of biogeochemical interfaces in soil. We investigated the role of these parental materials for the development of functional microbial guilds using the example of alkane-degrading bacteria harbouring the alkane monooxygenase gene (alkB) in artificial mixtures composed of different minerals and charcoal, sterile manure and a microbial inoculum extracted from an agricultural soil. We followed changes in abundance and community structure of alkane-degrading microbial communities after 3 and 12 months of soil maturation and in response to a subsequent 2-week plant litter addition. During maturation we observed an overall increasing divergence in community composition. The impact of metal oxides on alkane-degrading community structure increased during soil maturation, whereas the charcoal impact decreased from 3 to 12 months. Among the clay minerals illite influenced the community structure of alkB-harbouring bacteria significantly, but not montmorillonite. The litter application induced strong community shifts in soils, maturated for 12 months, towards functional guilds typical for younger maturation stages pointing to a resilience of the alkane-degradation function potentially fostered by an extant ‘seed bank’.
Similar content being viewed by others
Main
The tremendous microbial diversity in soils is, among other causes, a result of the huge structural heterogeneity and the enormous variety of biogeochemical interfaces (BGIs) present (Totsche et al., 2010). It has been postulated that during soil development different parental materials shape individual BGIs. They determine to a large extent the specific surface area and charge of BGIs and control for example, the water availability for microorganisms (Young and Crawford, 2004; Chorover et al., 2007). Such abiotic properties of BGIs may strongly influence microbial community structure and function. For instance, Vogel et al. (2014) demonstrated that organic carbon preferentially attaches to rough surfaces of mineral clusters, turning them into hotspots for microbial activity. In this study, we investigated the abundance and community structure of a functional microbial guild (alkane degraders harbouring the alkane monooxygenase gene alkB) at two different time points during the maturation of artificial soils formed from different parental materials. Thereby we compared artificial soils after a shorter period of maturation (namely, 3 months) and more developed soil structures (namely, 12 months of maturation), in each case before and after the addition of alkane-containing substrate (plant litter). We hypothesized that the functional guild studied will be largely influenced by the availability of alkane substrates, essential nutrients (for example, nitrogen) and water, which are largely controlled by differing sorption coefficients of the used parental materials that undergo an alteration process over time. Furthermore we wanted to understand if the ‘dormant or rare biosphere’ could act as a seed bank and ensure process stabilization after substrate addition (Giebler et al., 2013).
We used eight artificial soils (Pronk et al., 2012) of identical texture, yet differing in composition regarding the presence of clay minerals (montmorillonite (MT) and illite (IL), metal oxides (ferrihydrite (FH) and boehmite (B)) and charcoal (CH). These materials were chosen as representatives of common soil components with large and reactive surface areas, and can be expected to take part in the formation of BGIs in natural soils (Totsche et al., 2010). Initially, the development of microbial communities had been stimulated by adding a water-extracted fraction of microorganisms from an agricultural soil, classified as a Eutric Cambisol (Ultuna, Sweden) together with sterile manure to the sterile soil components (for details see Supplementary Material S1.1). We took soil samples after maturation phases of either 3 (T3) or 12 months (T12), in which we analysed the abundance (quantitative PCR, qPCR, Supplementary Material S1.2) and community structure (terminal restriction fragment length polymorphism analysis, T-RFLP, Supplementary Material S1.3) of alkane-degrading microbes, as well as response patterns towards the addition of plant litter from winter wheat after 2 weeks of incubation subsequent to maturation. Schulz et al. (2012) described the alkane monooxygenase gene (alkB) as a sensitive marker for the molecular analysis of alkane-degrading bacteria responding to subtle changes in substrate availability that is, fresh plant material, which in general contains large amounts of easily available alkanes in form of waxes. The litter was added on top of the microcosms to create a vertical substrate gradient. Thus, soil samples were taken from the litter–soil interface (top 1 mm of the soil column; T3-I, T12-I) and ‘bulk’ soil (10–11 mm of the soil column; T3-B, T12-B). All details on the Materials and Methods can be found in the Supplementary Material S1.
We detected higher alkB gene copy numbers in soils matured for 3 than in those matured for 12 months (Figure 1), regardless whether we analysed them before or after litter addition. Solely the soil where MT and CH had been added together exhibited stable alkB abundance patterns at both time points of maturation. However, the relative response towards litter addition was often stronger in soils with a longer maturation history (T12), particularly when soils contained illite mixtures or ferrihydrite (Figure 1 and Supplementary Material S2). The effects were more pronounced in samples from the litter–soil interface (T3-I and T12-I) than in bulk soil samples (T3-B and T12-B). A previous study revealed that the overall CO2 respiration rates for these artificial soils after 3 and 12 months of maturation were comparable (Pronk et al., 2012). Several studies therefore concluded that the amount of organic substrates and nutrients present was enough to support activity of the total microflora (Pronk et al., 2012; Babin et al., 2013; Pronk et al., 2013). However, the lower abundance of alkane degraders in soils, maturated for 12 months, in conjunction with the respective stronger response towards litter addition might reflect a depletion of easily bioavailable specific substrates (namely, alkanes) in these soils by degradation and/or maturation-driven reallocation of nutrients to smaller, poorly accessible soil structures (Kögel-Knabner et al., 2008; Pronk et al., 2012; Vogel et al., 2014).
Abundance patterns of alkB genes in artificial soils after 3 (T3) and 12 months (T12). Diamonds (left, y axis) represent alkB gene abundances in matured soils (T3 in black and T12 in grey; s.e. of n=3). Bars (right, y axis) show a relative comparison between the response patterns to litter addition after 12 months compared with 3 months in the different artificial soils (litter–soil interface: black pattern; bulk soil: grey pattern). A value of 1 indicates an identically strong response at T12 and T3 to litter addition, a value >1 indicates a stronger and a value <1 a weaker litter response at T12- compared with T3-litter treated samples. The response values were calculated as quotients of alkB gene abundances after litter treatment vs after maturation, but without litter (for detailed information on calculation see Supplementary Material S1.4; for absolute alkB gene copy numbers of all treatments and correlation with environmental factors see Supplementary Material S2).
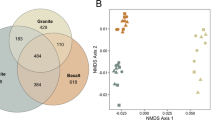
The community structure of alkB-harbouring bacteria diverged significantly between 3 and 12 months of soil maturation (before litter addition; Figure 2a) with strong differences in some of the dominant T-RFs (for example typically present at T3: 88, 95, 123, 266, 347, 360, 468, 469, 527; more specific for T12: 80, 82, 83, 106; for details see Supplementary Material S1.3 and S3). Moreover, the replicates of the different artificial soil mixtures showed higher variability at T12 when compared with T3 (that is, greater distance between triplicates in the non-metric multidimensional scaling (NMDS)-plot, Figure 2a; P=0.002, statistical calculation Supplementary Material S1.4). This may indicate a higher variability of the alkB community structure with longer maturation time, even within the same artificial soil mixture. When correlating the alkB community structure with the ‘soil complexity’ (that is, the artificial soils contained 1 versus 2 or 3 components in addition to texture-providing quartz), we found that indeed the ‘soil complexity’ significantly influenced the community structure after 3 and 12 months of maturation (P<0.05; Supplementary Material S4-D). Altogether these findings indicate an increasing heterogeneity of the BGIs with maturation time and/or soil complexity, which was accompanied by an increasing formation of microaggregates (Pronk et al., 2012). One may speculate, that the resulting higher hierarchical structure in more matured soils probably creates diverse niches favouring the development of diverging microbial communities in response to their particular microenvironment as also described by Vos et al. (2013).
Community structure of alkB genes in artificial soils illustrated by NMDS. Solid ellipses indicate 95%, dashed ellipses 99% of community spreads of T3 (red) or T12 (blue; for details see Supplementary Material S4). (a) community differences between matured soil without litter at T3 (open symbols) and T12 (closed symbols). The eight artificial soils are indicated by colour (for legend see a; n=1: MT and IL, n=2: FH, n=3: all others). Exemplary for selected soils, distances of replicates to their group centroid are represented by dashed lines, whereby greater distance indicates greater dissimilarity of community structure. (b) alkB community structure before (red or blue ellipse) and after litter addition (green ellipses). The blue arrow indicates strong community shift of T12. (c, d) show selected soil components significantly affecting the alkB communities in all T3 or T12 treatments, respectively (P<at least 0.05; for details see Supplementary Material S4).
The addition of fresh plant litter induced a pronounced shift of alkB community patterns in soils, maturated for 12 months (T12-I and T12-B compared with T12; Figure 2b, Supplementary Material S4), whereas no significant changes were observed after 3 months of maturation. Interestingly, the T12-I and T12-B communities after litter addition converged with those of the younger maturation stage, supporting the conclusion that the alkanes might have been depleted during the longer maturation without refeeding. The convergence points to the resilience of the alkane-degradation function through a potential ‘seed bank’ formed by dormant or rare alkane degraders in times of low substrate availability (Giebler et al., 2013). These microbes might be reactivated when the system experiences more favourable environmental conditions (Epstein, 2009; Shade et al., 2012). Their existence is furthermore indicated by high-abundant T-RFs formerly not detected in matured T12 soils (for example, T-RF 86, 88, 94, 95, 239, 463, 523 when comparing T12 with T12-I and T12-B; Supplementary Material S1.3 and S3).
When analysing the data sets of 3 and 12 months of maturation individually, the presence of illite and charcoal as soil components in the different soil mixtures were found to be the main factors controlling alkB community structure in all treatments (stronger significance for T3; Figure 2c and Supplementary Material S4) as also observed for the bacterial community in general (Ding et al., 2013). The role of clay minerals like illite might be to sequester large amounts of the soil organic matter (Kaiser and Guggenberger, 2003; Vogel et al., 2014) thereby affecting the nutrient availability (Pronk et al., 2013). The assumption of the reduced availability of nutrients after 3 months is furthermore supported by the significant impact of charcoal, which may adsorb nutrients and reduce their bioavailability (Sohi et al., 2010; Lehmann et al., 2011). A leaching of alkanes by the charcoal used in this study can be excluded (Pronk et al., 2012). However, the charcoal effect receded at later maturation stages, when the reactive sites of minerals and charcoal surfaces gradually became covered with organic matter (Pronk et al., 2012).
After 12 months of soil maturation, an emerging relevance of metal oxides as drivers for the community structure of alkB-harbouring microbes (Figure 2e) became evident. Metal oxides strongly interact with the organic matter of natural soils (Eusterhues et al., 2003; Kaiser and Guggenberger, 2003; Wagai and Mayer, 2007). However, a weak affinity and low sorption of organic matter had been reported for our artificial soils owing to constantly neutral pH (Heister et al., 2012; Pronk et al., 2013). Thus, factors other than nutrient availability might be responsible for the influence of metal oxides on the community structure as the increasing heterogeneity of the habitat creates more microniches. Potentially the noncharged surfaces of metal oxides in our experiment might function as valuable ‘safe havens’ still available for colonisation by microbes when other surfaces are already occupied. Thus, their role might become manifest when a soil reaches a higher hierarchical structure.
Overall, our results support the hypothesis that complex structured BGIs as generated by clay minerals or metal oxides and charcoal are important drivers for the development of microbial community structures and their functional traits. It seems that structure–diversity and structure–function relationships are emerging properties, which go beyond the characteristics of individual components, that is, they are the result of a dynamic interplay of the BGIs and microbial communities responding to these microhabitats.
References
Babin D, Ding GC, Pronk GJ, Heister K, Kögel-Knabner I, Smalla K . (2013). Metal oxides, clay minerals and charcoal determine the composition of microbial communities in matured artificial soils and their response to phenanthrene. FEMS Microbiol Ecol 86: 3–14.
Chorover J, Kretzschmar R, Garcia-Pichel F, Sparks DL . (2007). Soil biogeochemical processes within the critical zone. Elements 3: 321–326.
Ding G-C, Pronk GJ, Babin D, Heuer H, Heister K, Kögel-Knabner I et al. (2013). Mineral composition and charcoal determine the bacterial community structure in artificial soils. FEMS Microbiol Ecol 86: 15–25.
Epstein SS . (2009). Microbial awakenings. Nature 457: 1083.
Eusterhues K, Rumpel C, Kleber M, Kögel-Knabner I . (2003). Stabilisation of soil organic matter by interactions with minerals as revealed by mineral dissolution and oxidative degradation. Org Geochem 34: 1591–1600.
Giebler J, Wick LY, Chatzinotas A, Harms H . (2013). Alkane-degrading bacteria at the soil-litter interface: comparing isolates with T-RFLP-based community profiles. FEMS Microbiol Ecol 86: 45–58.
Heister K, Höschen C, Pronk G, Mueller C, Kögel-Knabner I . (2012). NanoSIMS as a tool for characterizing soil model compounds and organomineral associations in artificial soils. J Soils Sediments 12: 35–47.
Kaiser K, Guggenberger G . (2003). Mineral surfaces and soil organic matter. Eur J Soil Sci 54: 219–236.
Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S et al. (2008). Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J Plant Nutr Soil Sci 171: 61–82.
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D . (2011). Biochar effects on soil biota - A review. Soil Biol Biochem 43: 1812–1836.
Pronk GJ, Heister K, Ding G-C, Smalla K, Kögel-Knabner I . (2012). Development of biogeochemical interfaces in an artificial soil incubation experiment; aggregation and formation of organo-mineral associations. Geoderma 189–190: 585–594.
Pronk GJ, Heister K, Kögel-Knabner I . (2013). Is turnover and development of organic matter controlled by mineral composition? Soil Biol Biochem 67: 235–244.
Schulz S, Giebler J, Chatzinotas A, Wick LY, Fetzer I, Welzl G et al. (2012). Plant litter and soil type drive abundance, activity and community structure of alkB harbouring microbes in different soil compartments. ISME J 6: 1763–1774.
Shade A, Hogan CS, Klimowicz AK, Linske M, McManus PS, Handelsman J . (2012). Culturing captures members of the soil rare biosphere. Environ Microbiol 14: 2247–2252.
Sohi SP, Krull E, Lopez-Capel E, Bol R . (2010). A review of biochar and its use and function in soil. Adv Agron 105: 47–82.
Totsche KU, Rennert T, Gerzabek MH, Kögel-Knabner I, Smalla K, Spiteller M et al. (2010). Biogeochemical interfaces in soil: The interdisciplinary challenge for soil science. J Plant Nutr Soil Sci 173: 88–99.
Vogel C, Mueller CW, Höschen C, Buegger F, Heister K, Schulz S et al. (2014). Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils. Nat Commun 5: 2947.
Vos M, Wolf AB, Jennings SJ, Kowalchuk GA . (2013). Micro-scale determinants of bacterial diversity in soil. FEMS Microbiol Rev 37: 936–954.
Wagai R, Mayer LM . (2007). Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim Cosmochim Acta 71: 25–35.
Young IM, Crawford JW . (2004). Interactions and self-organization in the soil-microbe complex. Science 304: 1634–1637.
Acknowledgements
Funding by German Research Foundation in the framework of the priority programme 1315 ‘Biogeochemical Interfaces in Soil’ is greatly acknowledged. Funding of the ‘PILZNETZWERKE’ project by grants from the European Regional Development Fund and Saxony in an initiative of ‘Europe funds Saxony’ is acknowledged. For support in T-RFLP analysis, we are grateful to Katharina Wetzel and for help with NMDS-statistics to Ingo Fetzer. We thank the three anonymous reviewers of the original manuscript for their very helpful and constructive comments for improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Steinbach, A., Schulz, S., Giebler, J. et al. Clay minerals and metal oxides strongly influence the structure of alkane-degrading microbial communities during soil maturation. ISME J 9, 1687–1691 (2015). https://doi.org/10.1038/ismej.2014.243
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2014.243
This article is cited by
-
Soil phyllosilicate and iron oxide inhibit the quorum sensing of Chromobacterium violaceum
Soil Ecology Letters (2021)
-
Bacillus subtilis biofilm development in the presence of soil clay minerals and iron oxides
npj Biofilms and Microbiomes (2017)
-
Interaction of minerals, organic matter, and microorganisms during biogeochemical interface formation as shown by a series of artificial soil experiments
Biology and Fertility of Soils (2017)
-
Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls
Nature Communications (2016)