Abstract
To define the relationship between salivary SIgA antibodies and commensal oral bacteria, we examined the reactivity of SIgA antibodies from the saliva of four infants with their own colonizing Streptococcus mitis biovar 1 (S. mitis bv 1) clones (ribotypes). Immunoblot analysis was used to examine reactivity of these antibodies with persistent ribotypes isolated from the mouths of the infants over the first year postpartum. Results showed that the pattern of SIgA antibody reactivity with the majority of clones increased in complexity after colonization but that most additional bands were common to other clones, indicating that they represented shared antigens. However, unique bands were identified in 75% of the selected persistent clones. We hypothesized that if strain-specific SIgA antibody was induced in response to colonization of a particular clone and contributed to its elimination from the mouth, then the appearance of unique bands would immediately precede the disappearance of the strain. Seventy-three percent of all unique bands identified in the study fulfilled this criterion. Because the mouth is an open, dynamic environment and multiple factors are believed to play a role in the immune response at mucosal surfaces, it may not be possible to conclusively define the relationship between SIgA antibody and commensal bacteria. However, our data provide evidence that SIgA antibody, reactive with unique antigens of their own colonizing strains, is produced in infants and may point to a role of this antibody in regulating colonization by S. mitis bv 1.
Similar content being viewed by others
Introduction
The mucosal surfaces of the adult human comprise an area in the order of 400 m2 (Mestecky and McGhee, 1987). These barrier epithelia are, for the most part, delicate monolayers involved in the exchange of gases, the uptake of nutrients, excretion of waste products and other essential physiological functions. Such is the potential susceptibility of these surfaces to colonization and invasion by microbial pathogens that mammals and birds have evolved a sophisticated adaptive immune system, termed the mucosal immune system, to protect these surfaces. The mucosal immune system consists of sentinel secondary lymphoid tissue that is present at the portals of entry to the mucosae and is distributed throughout the aerodigestive tract, the genitourinary tract, eyes and mammary glands. Over 80% of the total number of B cells in humans are located in the alimentary canal and are committed to the synthesis of secretory immunoglobulin A (SIgA), the principal immunoglobulin isotype secreted onto mucosal surfaces. SIgA antibodies in the mucous layer and in exocrine secretions are considered to protect mucosal surfaces through immune exclusion, a noninflammatory mechanism that neutralizes toxins and facilitates the removal of microorganisms by the flow of secretions by blocking their adhesion to epithelial receptors and/or by agglutination (reviewed in Mestecky and McGhee, 1987).
However, despite the formidable commitment of the human immune system to SIgA synthesis, the mucosal surfaces are permanently colonized by complex microbiotas that appear to be unaffected by it (Bowden and Hamilton, 1998). It is not that the mucosal immune system fails to recognize commensal bacteria, as we and others have demonstrated salivary SIgA antibodies react with them (Smith et al., 1985, 1990; Gregory et al., 1990; Widerstrom et al., 1992; Cole et al., 1998, 1999, 2004), and commensal bacteria are known to be coated with SIgA (Brandtzaeg et al., 1968). It seems more likely that SIgA antibodies reactive with commensal bacteria lack the appropriate specificity and/or avidity to inhibit their adhesion (Quan et al., 1997). Consistent with this view, we previously examined the specificity of SIgA antibodies reactive with several species of viridans streptococci and Enterococcus faecalis that are commensals in the oropharynx and the large bowel, respectively, in whole saliva collected from human infants over the first 2 years of life (Cole et al., 1999). Cross-absorption showed that antigens common to the streptococci and enterococcus bound a significant amount of the antibody, although some species-specific antibodies were detected. Furthermore, we observed that although the proportion of SIgA antibody in whole saliva, reactive with the type strain of Streptococcus mitis biovar 1, increased from birth to 6 months, it declined over the next 18 months of life suggesting that the response was limited.
In parallel with our studies on the salivary mucosal immune response to commensal oral bacteria, we and others have examined the population dynamics of S. mitis biovar 1 in the mouth and have shown that, while this bacterium is stable at the species level, it exhibits significant clonal turnover and replacement (Hohwy and Kilian, 1995; Fitzsimmons et al., 1996; Hohwy et al., 2001; Kirchherr et al., 2005). Indeed, most of the clones in the current study were isolated at a single sampling visit and could not be re-isolated at the successive visit, 2 months later. However, a few clones persisted in the mouth for two successive visits or disappeared and reappeared at a later visit. We have termed these ‘persistent’ clones of S. mitis biovar 1 and have used them to examine the specificity of salivary SIgA antibodies, which react with them, before, during and after their appearance in the mouth. We hypothesized that the appearance of a clone in the mouth induces clone-specific SIgA antibodies that may play a role in its subsequent suppression or elimination.
Materials and methods
Study population
Details of the study population and selection criteria have been published previously (Fitzsimmons et al., 1994). Four healthy full-term infants from our cohort were included in the study. All four infants were breast fed for the first 3 months postpartum.
Whole-mouth saliva
Whole saliva and swabs of the mucosa and teeth, once erupted, were collected from the infants at 1–3 days, 1, 2, 4, 6, 8, 10 and 12 months postpartum. An additional sample was obtained at 2 weeks of age from one of the infants. Ethylene-diaminetetraacetic acid (EDTA) was added to all saliva samples to a final concentration of 5 mM to inhibit IgA1 protease activity and prevent the formation of calcium ion-dependent mucin–immunoglobulin complexes. Saliva was stored at −80 °C for subsequent immunological analysis.
Quantitation of secretory immunoglobulin A in whole-mouth saliva
To standardize the concentration of SIgA in each of the saliva samples used for immunoblotting (see below), total SIgA was quantified using an enzyme-linked immunosorbent assay (ELISA) as previously described (Fitzsimmons et al., 1994) with minor modifications. The ELISA was performed in 96-well Immulon II microtiter plates (Dynatech Laboratories Inc., Chantilly, VA, USA) using an assay volume of 100 μl. With the single exception of the antibody to human secretory component obtained from Dako (Carpinteria, CA, USA), all antibodies employed in the ELISA were affinity purified IgG antibodies obtained from Jackson ImmunoResearch (West Grove, PA, USA). All antibody incubation steps were conducted for 1 h at room temperature on an orbital shaker (Bellco, Vineland, NJ, USA). During antibody incubation steps, wells were washed three times with phosphate-buffered saline (PBS) buffer, pH 7.4, containing 0.1% Tween 20 (PBS-T) using a Columbus plate washer (Tecan, Durham, NC, USA). Briefly, four dilutions of each saliva sample were prepared in PBS-T. For saliva samples collected between birth and 4 months of age, the dilutions ranged from 1:50 to 1:800. Dilutions of samples collected from infants between 6 and 12 months of age ranged from 1:600 to 1:2000. Wells were coated with rabbit anti-goat γ chains at a concentration of 3 μg ml−1 in 0.05 M carbonate buffer, pH 9.6, containing 0.02% NaN3, overnight at 4 °C. This served as a presenting antibody. Following washing, wells were blocked with PBS-T, pH 8.0, containing 0.1% bovine serum albumin. Goat anti-human α chains at a concentration of 2.4 μg ml−1 were added to the wells as the capture antibody. Saliva dilutions were added to the wells in triplicate and incubated for 1 h at room temperature. After washing, horseradish peroxidase-conjugated rabbit anti-human secretory component at a dilution of 1:5000 was added as the reporter antibody. Following washing, captured SIgA was detected using 1 mg of o-phenylenediamine per ml of citrate–phosphate buffer, pH 4.5, containing 0.012% hydrogen peroxide. Absorbance was measured at 450 nm using a Spectrum Rainbow microtiter plate reader (Tecan, Durham, NC, USA). Dilutions of authentic colostral SIgA (MP Biomedicals/Cappel, Solon, OH, USA) of known concentration were included on each plate to create a standard curve, from which SIgA concentration in saliva samples was determined by interpolation. Adult human whole saliva of known SIgA concentration from our laboratory was used as a positive control. Appropriate background and negative controls were run on every plate.
Swabs
Swab (BBL CultureSwab, Collection and Transport System, Becton Dickinson Co., Sparks, MD, USA) samples were taken from the left and right buccal mucosa, the tongue and the labial surfaces of the lower and upper incisors after eruption. Processing of the swabs has been described in detail previously (Pearce et al., 1995). Briefly, the swabs were transported to the laboratory, where their heads were cut off into a reduced transport fluid. Bacteria were released from the swab head by ultrasound and the dispersed sample was serially diluted to 10−5. Aliquots of the dilutions were inoculated onto trypticase soy agar containing 5% sheep blood, using a spiral plater (Autoplate 4000, Spiral Biotech, Cincinnati, OH, USA)
Isolation and identification of S. mitis biovar 1
Isolation and identification of S. mitis biovar 1 have been described in detail previously (Pearce et al., 1995). Briefly, α-hemolytic, Gram-positive, catalase-negative cocci were randomly picked from sheep-blood agar plates incubated at 37 °C for 48 h in a candle extinction jar. S. mitis was presumptively identified by latex agglutination using pooled polyclonal rabbit antisera raised against the homologous species and absorbed to remove activity against other species of viridans streptococci. Identity was confirmed by use of a panel of physiological tests and by partial sequencing of the sodA gene from random isolates.
Ribotyping S. mitis biovar 1 isolates
The method employed has been described in detail previously (Fitzsimmons et al., 1996; Kirchherr et al., 2005). Briefly, genomic DNA was extracted, purified and restricted with PvuII. The digested DNA was separated by horizontal gel electrophoresis using 0.8% agarose and transferred to nylon membranes. The membranes were hybridized with digoxigenin-labeled cDNA probes synthesized by using 16S and 23S rRNA from Escherichia coli as a template. The hybridized digoxigenin-labeled cDNA probes were detected by incubation with anti-digoxigenin Fabγ fragments conjugated with alkaline phosphatase and development with nitro blue tetrazolium and X-phosphate. To ensure that isolates with matching PvuII ribotypes were identical, the Sall digests of these isolates were also ribotyped. Only isolates with matching PvuII and Sall ribotypes were considered to represent the same clone.
Selection of ribotypes and saliva
Out of a total of 568 ribotypes recovered during the study from the four infants, only 30 were isolated from the mouth at more than one sampling time. These ribotypes were termed ‘persistent’. Because of limited volumes of saliva and the low levels of SIgA in infant saliva, it was not possible to study every persistent ribotype. Those selected for study are shown in Figure 1. Antigen extracts (see below) from these persistent ribotypes were reacted with the saliva from the infant from whom they were isolated. Each antigen extract was reacted with the saliva collected at the visits prior to the ribotype isolation, during the ribotype isolation and, whenever possible, immediately after the ribotype was no longer detected.
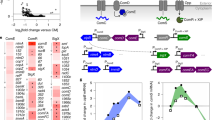
Persistence patterns of selected ribotypes in the oral cavity. Ribotype numbers are listed to the left of the y-axis. The first digit of each ribotype number identifies the infant. The following numbers identify the ribotype. Solid horizontal lines indicate the age of the infant at the time(s) the ribotype was isolated. The interrupted line indicates periods when the ribotype was not isolated. The surface from which each ribotype was isolated is indicated as D (upper and lower central incisors), T (dorsum of the tongue) or B (buccal mucosa). Ribotypes represented by more than a single isolate are denoted by bold solid horizontal lines.
S. mitis biovar 1antigen extraction
Selected ribotypes were inoculated into tubes containing 10 ml of Todd Hewitt Broth (Bacto, Difco Laboratories obtained from Becton Dickinson and Co.) and grown for 48 h in 5% CO2. Cell surface extracts were prepared by a method that did not disrupt the cells (Pearce et al., 1995). Briefly, cells were sedimented by centrifugation, washed twice in 10 mM HEPES buffer, pH 7.4, re-suspended in 0.5 ml of the same buffer and held on ice. Cell suspensions were then subjected to four 1-minute cycles of ultrasound at 80 W using a Branson Sonifier 250 (Branson Ultrasonics Corp., Danbury, CT, USA). After sonication, cells were sedimented by centrifugation and stained using Gram's method to confirm that they had not been ruptured and their walls had remained intact. Supernatants from the sonicates were assayed for total protein using the BCA protein assay (Pierce Biotechnology Inc., Rockford, IL, USA), according to the manufacturer's instructions, and then stored at −20 °C. The same antigen extract from each ribotype was used throughout the study to avoid any variation in antigen composition.
Protein profiles of S. mitis biovar 1 extracts
Extracts were separated by SDS-polyacrylamide gel electrophoresis using a MiniProtean II System (Bio-Rad Laboratories, Hercules, CA, USA). Samples containing 10 μg of protein were boiled for 10 min in sample buffer and loaded into wells of 5% stacking/12% running gels. Electrophoresis was performed in Tris–glycine–SDS buffer for 1 h at 200 V. The gels were stained with Coomassie brilliant blue R-250 and imaged using a gel imager (AlphaImager HP, Alpha Innotech Corporation, San Leandro, CA, USA).
Reproducibility of extracts
We examined the reproducibility of the extraction method in two ways. We grew several independent batches of two different ribotypes of S. mitis. Washed cells from these batches were extracted and proteins separated as described above. We compared different extracts of the same ribotype, ran both on a single gel and on different gels on different days. Analysis performed within the BioNumerics program (see below) showed that the variation between multiple extracts of the same ribotype that was run together on a single gel was <3.0% and that on different gels at different times was <7.0%.
Western immunoblotting
Five micrograms of extract were separated by SDS-polyacrylamide gel electrophoresis as described above and transferred to Immobilon-P polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA) using a Trans-Blot SD electrophoretic transfer cell (Bio-Rad Labs.). Membranes were stained with Ponceau-S to visualize bands, cut into individual strips and de-stained in water. Strips were blocked for 2 h in 4% bovine serum albumin and incubated overnight with saliva samples diluted in PBS-T, to contain a concentration of 1.5 μg of SIgA per ml. Each strip was incubated with 2 ml of diluted saliva. Biotin-conjugated rabbit IgG antibody to human α chains (Jackson ImmunoResearch) was used to detect bound SIgA. Strips were incubated with horseradish peroxidase-conjugated streptavidin (Jackson ImmunoResearch) and developed with 0.5 mg of 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St Louis, MO, USA) per ml of 0.01 M PBS, pH 7.4, containing 0.01% H2O2. Sterile deionized water was used to stop the reaction and strips were allowed to dry completely before images were recorded using a flatbed scanner (Hewlett-Packard, Palo Alto, CA, USA).
Analysis of band patterns
Images of SDS-polyacrylamide gel electrophoresis profiles and western immunoblot band patterns were imported into the BioNumerics program (Applied Maths, Sint-Martens-Latem, Belgium). Cluster analysis and band-matching analysis were performed using the ‘Different Bands’, band-based similarity coefficient. Band matching was used to divide bands into classes of common bands. Position tolerances for cluster analysis and band-matching functions were set at 5% tolerance and 3% optimization. Dendrograms were constructed using unweighted pair group method with arithmetic mean (UPGMA). The polymorphism analysis tool was used to create binary tables, based on band presence or absence. In this way, unique and common bands were identified among samples and samples were then sorted according to visit, ribotype or discriminative characters.
Results
Patterns of colonization by persistent ribotypes
Individual ribotypes demonstrated several patterns of persistence within the oral cavity and those studied are shown in Figure 1. Ribotypes 6.147, 8.47 and 8.122 were found on the same oral surfaces on every occasion they were isolated from the mouth, the latter ribotype being present both on the tongue and the buccal mucosa. The other 13 persistent ribotypes were isolated from different surfaces on each sampling occasion. Six potential patterns of colonization of surfaces by these ribotypes were possible. The isolates could initially colonize the teeth (D) and then go to the tongue (T), or buccal mucosa (B), or go from T to D or from T to B, or go from B to D or from B to T. The ribotypes shown in Figure 1 included representatives of each of these patterns except D to T, which was not observed in any infant in the study, and T to D, which was observed only in infant no. 6 on visits, for which saliva was not available. The majority of ribotypes colonizing two surfaces were initially isolated from the buccal mucosa (B) and later from another surface. Interestingly, very few ribotypes were found to move from the tooth surface (D) to the buccal mucosa (B) or tongue (T).
Persistent ribotypes also varied temporally in terms of colonization patterns. Some ribotypes were isolated from the mouth on adjacent visits (for example, ribotypes 3.6, 6.5, 8.47 and 10.62), whereas others were isolated from samples taken up to 10 months apart (for example, 3.83, 6.88, and 8.16). An interesting observation was that more than one isolate of a given ribotype was isolated from some samples (Figure 1). Of the persistent ribotypes 3 of 16 fit this description (6.56, 8.16, 10.14 and 10.62).
Protein profiles of cell extracts of ‘persistent’ S. mitis biovar 1 ribotypes
Before analyzing the specificity of salivary SIgA antibodies, reactive with the selected ribotypes of S. mitis biovar 1, we determined the extent of variation in the extracts among the isolates. Cluster analysis revealed better than 97% similarity among protein profiles of isolates of the same ribotype when run on the same gel (data not shown). Thus, differences in the western immunoblot antibody banding patterns were most likely to reflect variation in antibody specificity rather than variation in protein composition among antigen extracts from different ribotypes.
Complexity of antibody reactivity patterns over the first year postpartum
As an example of the immunoblot patterns obtained in the study, panel A of Figure 2 shows the protein profile of clone 6.5 and four immunoblot strips of clone 6.5 reacted with whole saliva collected at 1, 2, 4 and 6 months of age. Panel B shows the detection of bands in these lanes by the BioNumerics program. From our previous studies (Cole et al., 1999) we would expect a number of common bands to be recognized for every ribotype. These bands may represent binding by genus or species-specific antibody. Overall, 38 bands were identified in the profiles of the 16 ribotypes. Eighty-seven percent of these were bands found in at least one of the profiles of more than one ribotype. Therefore, the majority of bands identified in this study were common bands shared among/between ribotypes. Within each infant, some bands were detected in all ribotypes examined, although not necessarily in all profiles. Salivary SIgA antibodies from infant no. 3 reacted with nine such bands, infant no. 6 with five bands, infant no. 8 with 10 bands and infant no. 10 with 6 bands (Figures 3 and 4). One band (109.0 kDa) was recognized in every ribotype of every infant included in the study. Four other bands (39.0, 47.5, 50.5 and 67.0 kDa) were recognized in all but one of the ribotypes of every infant examined.
The fine specificity of salivary SIgA antibodies in four infants during the first year postpartum was determined by immunoblotting. A non-disruptive cell extract from each ribotype was separated by SDS-PAGE and transferred to a PVDF membrane. Strips (lanes) were incubated with whole saliva and bound SIgA antibodies were detected using anti-human α-chain antibodies. Binary band matching tables were constructed based on the presence or absence of bands. Each block represents a band. Rows of bands represent common band classes. The molecular masses of the bands of the profiles of the ribotypes from a single infant were compared and the patterns were grouped by the age of the infant (descending age from left to right). Grouping by the age of the infant allows detection of common bands (antigens) shared among ribotypes. Antibodies reactive with these common antigens could have been induced by previous colonization by other ribotypes. Each column represents the immunoblot profile of individual persistent ribotypes isolated from each infant and reacted with saliva collected over time. The identity of the ribotype and age (months) of the infant at which the saliva was collected are indicated at the bottom of each column.
Band-matching analysis with samples sorted by ribotype. Binary band-matching tables were constructed based on the presence or absence of bands. Each block represents a band. Rows of bands represent band classes. In this figure, for each infant, the columns represent groups of immunoblot profiles of each persistent ribotype reacted with saliva collected over time, that is, before, during and after the ribotype was detected in the mouth. Arraying the data in this manner allows visualization of the increasing complexity of immunoblot pattern that follows colonization of the ribotype. The identity of the ribotype and the age of the infant (months) when the saliva was collected are indicated at the bottom of each column.
Saliva collected from all infants before 2 months of age showed low reactivity (0–5 bands) for each of the ribotypes. For the majority of persistent ribotypes, the numbers of bands recognized by salivary SIgA antibody in the immunoblot profiles increased from the visit preceding the initial isolation until the visit at which the ribotype was no longer detected (Table 1). This pattern was seen for 11 ribotypes (3.6, 3.54, 3.60, 3.83, 6.5, 6.72, 6.147, 8.47, 10.14, 10.62 and 10.94), however, there were some exceptions. Although the profiles of ribotypes 6.56, 6.88 and 6.123 were complex with 10–18 bands, they did not show an increase in numbers of bands following isolation of the organism. Also, ribotypes 8.16 and 8.122 with fewer bands in their profiles, showed a relatively stable number of bands over time.
The molecular masses of the bands in the profiles of the ribotypes from a single infant were then compared and the patterns, grouped by the age of the infant, are shown in Figure 3. Grouping by the age of the infant allows easy detection of common bands in an immunoblot profile of a ribotype that could be the result of previous colonization by another ribotype. Thus, in infant no. 6, of the 17 bands (Table 1), which were detected by SIgA antibody in saliva, collected prior to isolation of ribotype 6.56, only 3 had not been detected in profiles of ribotypes that had colonized previously (Figure 3).
It was also possible to recognize unique bands that were detected in one or more of the profiles of an individual ribotype. These were generally detected either at colonization or post colonization. However, in the case of infants no. 6 and no. 10, unique bands in a profile were detected prior to isolation of the organism. The unique bands and the time when they were detected are shown in Table 2. Generally, the unique bands were detected by SIgA antibodies in saliva, collected at one sampling visit. The exceptions to this observation were interesting in that the band was detected by SIgA antibodies in saliva samples, collected at several visits. Thus, the 17.0 kDa band in the immunoblot profile of ribotype 6.147 was recognized by SIgA antibody in saliva, collected at 1, 2, 10 and 12 months. In infant no. 10, unique bands detected in ribotype 10.62 before colonization persisted in the profiles from 1 to 8 months. Unique bands were not detected in the profiles of ribotypes 3.60, 3.83, 6.72 and 6.88.
Discussion
Previously, we have performed longitudinal studies of SIgA antibodies that react with S. mitis biovar 1 in saliva of neonates and infants over the first 2 years of life (Cole et al., 1999). We detected SIgA antibodies reactive to S. mitis but also with the oral bacterium S. mutans and the commensal enteric bacterium E. faecalis, even though these bacteria were never detected in the mouths of the infants. Furthermore, by cross-absorbing saliva with S. mitis, S. oralis, S. mutans and E. faecalis, we determined that the vast majority, but not all, of SIgA antibodies reactive to S. mitis were, in fact, directed to antigens common to these four species. In addition, even though the total concentration of SIgA in saliva increased from birth to 24 months of age, S. mitis-reactive SIgA antibodies constituted a decreasing proportion of SIgA over this period of time. Considering our data and those of others showing extensive clonal turnover and replacement of S. mitis (Hohwy and Kilian, 1995; Fitzsimmons et al., 1996; Hohwy et al., 2001; Kirchherr et al., 2005), we hypothesized that the concentration of S. mitis-specific SIgA antibody in saliva is limited because the mucosal immune system has a very short exposure to the individual clones of S. mitis that appear and rapidly disappear from the mouth. Under these conditions, it might be expected that the secretory immune system would respond to those antigens that are common to every clone of S. mitis because these provide a ‘stationary target’. Such antigens are likely to be species-specific, genus-specific or even extended to related genera.
We took advantage of the fact that we were able to isolate a limited number of S. mitis biovar 1 ribotypes, from our study cohort, that persisted from one visit to the next or reappeared in the mouth on the same or a different oral surface after an interval of several visits. By comparing the reactivity of salivary SIgA antibodies with these ribotypes before, during and after their appearance in the mouth, we were able to determine if the appearance of a ribotype was recognized by the mucosal immune system and if it was associated with the induction of ribotype-specific SIgA antibodies. We are aware of the limitations of our approach that compared banding patterns of immunoblots, in that we were unable to tell if there is variation in the complement of epitopes of proteins that migrate identically on different immunoblot strips. Furthermore, we were constrained by the low number of persistent clones and the small volumes of saliva that can be obtained from human neonates.
The simple patterns of immunoblots developed with saliva samples collected before 2 months of age (Table 1) suggest that neonates produce only a narrow repertoire of SIgA antibodies in response to the limited diversity of pioneer bacteria colonizing the mouth, of which S. mitis is the dominant species. It is possible that antigens specific for ribotypes that colonize shortly after birth do not provide a sufficient stimulus to the mucosal immune system. Early colonizing strains may instead induce antibodies directed against common antigens that contribute to their elimination, precluding induction of SIgA antibodies to unique antigens. With increasing age, we showed, in most cases, an increase in the numbers of bands recognized by SIgA antibodies. Presumably this reflects the broadening specificities of SIgA antibodies induced by the expanding population of S. mitis biovar 1 and other related bacteria that colonize the infant over time (Table 1).
The detection of bands unique to a particular ribotype provides support for the generation of ribotype-specific antibody. However, there is no direct evidence that the antibody reactive to these bands was induced by that ribotype, instead it may have been induced by other undetected ribotypes or other related bacteria colonizing the infant. However, these antibodies did not react with bands common among the persistent ribotypes in an infant. Therefore, they discriminated among the ribotypes colonizing the infant and any influence they had on colonization would affect a specific ribotype.
Some unique bands were only demonstrated by SIgA antibody in single saliva samples collected at colonization or post colonization. This was the case for 9 of the 12 ribotypes (Table 2). It could be suggested that colonization by the ribotype induced SIgA antibodies specific for bands that were unique among the other persistent ribotypes, isolated from the infant, and that these antibodies resulted either in the elimination of the ribotype from the site or caused its numbers to fall below our ability to detect it. That the antibody to ‘unique’ bands was very often transient in nature is consistent with the loss of the organism from the site eliminating the antigenic stimulus.
In contrast, the preponderance of the immunoblot profiles comprised common bands that were detected over several months. The antigens that induce this antibody may be derived from colonizing strains that are stable and/or antigens common among transient strains. The outcome is that the antibodies persist in the saliva. In contrast, the antibodies reactive to 28 of 36 unique bands were demonstrated only once. This finding argues against the suggestion that other colonizing bacteria stimulated the antibodies that detected these antigens and supports the view that the colonizing ribotype induced ribotype-specific antibody.
If other strains of S. mitis biovar 1 or related organisms colonizing the infant were the stimulus for the antibodies that detected unique bands, then the antibodies cannot be regarded as ribotype or strain specific. However, although these antibodies may recognize antigens that are carried by S. mitis and other colonizing bacteria, they can be described as antibodies that discriminate among S. mitis ribotypes. Thus, they may define antigenic groups of S. mitis strains. Despite their lack of precise specificity, they may function in a manner similar to ribotype-specific antibody by interacting only with specific strains among the oral population of S. mitis biovar 1. As mentioned above, the ‘loss’ of a ribotype from a site could be the result of SIgA antibodies directed against adhesins, although the transient nature of the response would allow the ribotype to recolonize, perhaps from an associated habitat.
In the current study, we have demonstrated that ribotypes of S. mitis biovar 1 colonizing an infant's mouth are exposed to a diverse repertoire of SIgA antibodies. Many recognize common bands but a relatively small number, induced either by the colonizing ribotype or bacteria with cross-reacting antigens, react with bands unique to a persistent ribotype colonizing an infant. The antibodies directed against these ‘ribotype-specific’ antigens may be a significant force in removing the organism from a site. Identification and characterization of these antigens could contribute to an understanding of the role played by mucosal immunity in clonal turnover of human commensal bacteria.
References
Bowden GH, Hamilton IR . 1998. Survival of oral bacteria. Crit Rev Oral Biol Med 9: 54–85.
Brandtzaeg P, Fjellanger I, Gjeruldsen ST . 1968. Adsorption of immunoglobulin A onto oral bacteria in vivo. J Bacteriol 96: 242–249.
Cole MF, Bryan S, Evans MK, Pearce CL, Sheridan MJ, Sura PA et al. 1998. Humoral immunity to commensal oral bacteria in human infants: salivary antibodies reactive with Actinomyces naeslundii genospecies 1 and 2 during colonization. Infect Immun 66: 4283–4289.
Cole MF, Bryan S, Evans MK, Pearce CL, Sheridan MJ, Sura PA et al. 1999. Humoral immunity to commensal oral bacteria in human infants: salivary secretory immunoglobulin A antibodies reactive with Streptococcus mitis biovar 1, Streptococus oralis, Streptococcus mutans, and Enterococcus faecalis during the first two years of life. Infect Immun 67: 1878–1886.
Cole MF, Evans MK, Kirchherr JL, Sheridan MJ, Bowden GH . 2004. Study of humoral immunity to commensal oral bacteria in human infants demonstrates the presence of secretory immunoglobulin A antibodies reactive with Actinomyces naeslundii genospecies 1 and 2 ribotypes. Clin Diagn Lab Immunol 11: 473–482.
Fitzsimmons SP, Evans M, Pearce C, Sheridan MJ, Wientzen R, Bowden G et al. 1996. Clonal diversity of Streptococcus mitis biovar 1 isolates from the oral cavity of human neonates. Clin Diagn Lab immunol 3: 517–522.
Fitzsimmons SP, Evans MK, Sheridan MJ, Wientzen R, Cole MF . 1994. Immunoglobulin A subclasses in infant′s saliva and in saliva and milk from their mothers. J Pediatr 124: 566–573.
Gregory RL, Kindle JC, Hobbs LC, Filler SJ, Malmstrom HS . 1990. Function of anti-Streptococcus mutans antibodies: inhibition of virulence factors and enzyme neutralization. Oral Microbiol Immunol 5: 181–188.
Hohwy J, Kilian M . 1995. Clonal diversity of the Streptococcus mitis population in the human oral cavity and pharynx. Oral Microbiol Immunol 10: 19–25.
Hohwy J, Reinholdt J, Kilian M . 2001. Population dynamics of Streptococcus mitis in its natural habitat. Infect Immun 69: 6055–6063.
Kirchherr JL, Bowden GH, Richmond DA, Sheridan MJ, Wirth KA, Cole MF . 2005. Clonal diversity and turnover of Streptococcus mitis bv. 1 on shedding and nonshedding oral surfaces of human infants during the first year of life. Clin Diagn Lab Immunol 12: 1184–1190.
Mestecky J, McGhee JR . 1987. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol 40: 153–245.
Pearce C, Bowden GH, Evans M, Fitzsimmons SP, Johnson J, Sheridan MJ et al. (1995). Identification of pioneer viridans streptococci in the oral cavity of human neonates. J Med Microbiol 42: 67–72.
Quan CP, Berneman A, Pires R, Avrameas S, Bouvet J-P . 1997. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun 65: 3997–4004.
Smith DJ, King WF, Taubman MA . 1990. Salivary IgA antibody to oral streptococcal antigens in predentate infants. Oral Microbiol Immunol 5: 57–62.
Smith DJ, Taubman MA, Ebersole JL . 1985. Salivary IgA antibody to glucosyl-transferase in man. Clin Exp Immunol 61: 416–424.
Widerstrom L, Bratthall D, Hamberg K . 1992. Immunoglobulin A antibody activity to mutans streptococci in parotid, submandibular and whole saliva. Oral Microbiol Immunol 7: 326–331.
Acknowledgements
This work was supported by Public Health Service Grant DE08171 from the National Institute of Dental and Craniofacial Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wirth, K., Bowden, G., Kirchherr, J. et al. Humoral immunity to commensal oral bacteria in human infants: evidence that Streptococcus mitis biovar 1 colonization induces strain-specific salivary immunoglobulin A antibodies. ISME J 2, 728–738 (2008). https://doi.org/10.1038/ismej.2008.26
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2008.26