Abstract
Microbial fuel cells (MFCs) have the potential to combine wastewater treatment efficiency with energetic efficiency. One of the major impediments to MFC implementation is the operation of the cathode compartment, as it employs environmentally unfriendly catalysts such as platinum. As recently shown, bacteria can facilitate sustainable and cost-effective cathode catalysis for nitrate and also oxygen. Here we describe a carbon cathode open to the air, on which attached bacteria catalyzed oxygen reduction. The bacteria present were able to reduce oxygen as the ultimate electron acceptor using electrons provided by the solid-phase cathode. Current densities of up to 2.2 A m−2 cathode projected surface were obtained (0.303±0.017 W m−2, 15 W m−3 total reactor volume). The cathodic microbial community was dominated by Sphingobacterium, Acinetobacter and Acidovorax sp., according to 16S rRNA gene clone library analysis. Isolates of Sphingobacterium sp. and Acinetobacter sp. were obtained using H2/O2 mixtures. Some of the pure culture isolates obtained from the cathode showed an increase in the power output of up to three-fold compared to a non-inoculated control, that is, from 0.015±0.001 to 0.049±0.025 W m−2 cathode projected surface. The strong decrease in activation losses indicates that bacteria function as true catalysts for oxygen reduction. Owing to the high overpotential for non-catalyzed reduction, oxygen is only to a limited extent competitive toward the electron donor, that is, the cathode. Further research to refine the operational parameters and increase the current density by modifying the electrode surface and elucidating the bacterial metabolism is warranted.
Similar content being viewed by others
Introduction
Applications of microbial fuel cells (MFCs) promise energy-efficient conversion of dissolved organics and electron donors and even the generation of useful carbon neutral power (Rabaey and Verstraete, 2005). Certainly in the context of energy efficient wastewater treatment, they have received considerable attention (Liu et al., 2004; Rabaey et al., 2005c). The process that drives them, extracellular electron transfer, has in recent years shown high versatility in the type of conversions that can be achieved (Rabaey et al., 2007). Therefore, a broader array of applications is emerging, ranging from cathode-driven denitrification (Clauwaert et al., 2007a) to anode driven sulfide removal (Rabaey et al., 2006). To underline their versatility beyond energy generation, MFCs should rather be designated ‘Bio-Electrochemical Systems’ (Rabaey et al., 2007). In Bio-Electrochemical Systems, bacteria have been found to deploy several strategies to use electrodes as electron acceptors. A direct contact is established either through membrane-bound complexes (Bond and Lovley, 2003) or through conductive nanowires (Reguera et al., 2005; Gorby et al., 2006), or bacteria produce or use soluble components as electron carriers (Rabaey et al., 2005a). Those processes have been extensively studied for electrons flowing away from bacteria. But how bacteria take in electrons from insoluble donors has not been established yet, despite a rapidly increasing number of studies on biocatalyzed cathodes (Bergel et al., 2005; He and Angenent, 2006). In two studies on cathode-driven bacterial reduction, low cathodic potentials were applied at which hydrogen gas could evolve at the cathode (Gregory et al., 2004; Thrash et al., 2007). However, the capacity by a hydrogen uptake deficient Geobacter strain to receive electrons indicates that direct transfer may be possible (Gregory et al., 2004). Recent studies demonstrate that cathode-driven growth is possible at high cathodic potentials (around and above 0 mV) (Clauwaert et al., 2007a, 2007b). This potential range is important as it indicates that the process is unlikely to occur through cathodic hydrogen generation and subsequent bacterial hydrogen consumption. The understudied cathodic process may play a crucial role in the sustained operation of MFCs, in addition to delivering needed insight into the oxidation of insoluble electron donors in circumneutral conditions and mesophilic conditions. Currently, the cathode is the main impediment to the application of MFC-based generation of electricity.
This study therefore aimed to (i) develop a loop-based biocathode, capable of directly reducing oxygen, (ii) characterize the community responsible for the cathode catalysis and (iii) obtain pure cultures and study the biocathode properties of those isolated bacteria.
Methods
Microbial fuel cell construction
The MFCs had the same anode design as described previously (Aelterman et al., 2006) and were filled with granular graphite (El Carb 100, Graphite Sales Inc., Chagrin Falls, OH, USA). The anode had a total volume of 336 ml, and after filling with granules the liquid volume was 151 ml. A cation exchange membrane (Ultrex, Membranes International Inc., Glen Rock, NJ, USA) was used to separate the anode and the cathode. To construct the cathode, a frame was bolted against the membrane, which allows the cathode to be clamped tightly to the membrane. The cathodic electrodes used in these experiments were graphite felt (Morgan Industrial Carbon, Caboolture, Queensland, Australia) and woven carbon fibers (Fiberglast, Brookville, OH, USA). The cathodes had a total thickness of 2 mm and the carbon fiber cathode was tightly fixed and pushed against the membrane with 3 mm mesh size stainless steel mesh (Locker, Dandenong, Victoria, Australia), which functioned as current collector. The cathode size was 12 × 14 cm, creating a cathode projected surface of 168 cm2. The graphite felt did not require a current collector because of its higher conductivity. An Ag/AgCl reference electrode (Bio-Analytical, West Lafayette, IN, USA) was inserted in the anode compartments, its potential was assumed to be 198 mV versus standard hydrogen electrode (SHE) (this value may differ slightly relative to temperature and ionic strength fluctuations).
Reactor operation
Two MFCs were operated in parallel, either with a carbon fiber cathode (R1) or a graphite felt cathode (R2), at start-up. Both cathode surfaces were moistened with modified M9 medium at 4 l h−1 (Rabaey et al., 2005c) devoid of electron donor. The anode was fed continuously with modified M9 medium at a rate of 0.55 l d−1 and had a 4 l h−1 recirculation. 1 g l−1 sodium acetate was added to the anode feed. The cathodes were inoculated with a mixture of environmental samples obtained from rusted metal poles in the Brisbane River at a freshwater section, sediment from a pond at the University of Queensland and mixed liquor from a domestic activated sludge plant (Luggage Point WWTP). The external resistance was adapted over time to allow the generation of more current, as a maximum of 100 Ω was used. The pH was measured in the cathode liquid using a GPH Electroder pH electrode branched to an EZIDO PL-500 acquisition unit (Extech, Melrose, MA, USA). After 48 days of operation, the effluent of the anode was used as influent and recirculation liquid for the cathodes (the loop based system), rather than the prepared medium. The excess catholyte was removed daily. This operation was continued for another 35 days until day 83, after which reactor samples were taken. The electrode of R1 was split in three, of which one-third was sacrificed for sampling, the other two-thirds were split over the two reactors and new carbon fiber was used to complete the electrodes. This created two reactors with carbon fiber cathodes. The reactor with the new electrodes was operated for another 129 days in the same loop type mode, bringing the total experiment time to 212 days.
Electrochemical monitoring and data representation
Measurements were performed according to previous reports (Rabaey et al., 2005b) (Logan et al., 2006). At every 60 s, the voltage over the MFC was recorded using an Agilent HP34970 data acquisition unit. Potentiostatic measurements and controls were performed using a PAR VMP-3 Potentiostat (Princeton Applied Research, Oak Ridge, TN, USA). The polarization curves were recorded by operating the MFCs in open circuit for 2 h, after which the cell voltage was decreased to 0 mV at a rate of 0.2 mV s−1 for the mixed population and increased again to the open circuit voltage (OCV). For the pure cultures, the scan rate was 1 mV s−1 and the OCV period 30 min to avoid too long measurement periods (Aelterman et al., 2006).
Clone library analysis
DNA was extracted from the MFC samples, scraped from the cathode of R1 on day 83, using the bead-beating method in conjunction with the BIO 101 FastDNA spin kit for soil (QBiogene, Seven Hills, New South Wales, Australia) as per the manufacturer's instructions. The DNA concentration was assessed by a low molecular mass DNA ladder (Fermentas, Quantum Scientific, Murarrie, Queensland, Australia) on a 1% agarose gel. Amplification of bacterial 16S rRNA genes was achieved using primers 63f and 1387r (Marchesi et al., 1998). PCRs contained 200 ng μl−1 of each primer (Sigma, Castle Hill, New South Wales, Australia), 1 μl of DNA template, × 10 reaction buffer (Abgene, Willoughby, New South Wales, Australia), 25 mM MgCl2, 200 μM of each dNTP and 1 U Red Hot Polymerase (Abgene) in a 50 μl reaction. Thermocycling parameters were: −94 °C denaturation for 10 min followed by 35 cycles of 94 °C for 1 min, 55 °C annealing for 1 min and a 74 °C extension for 1 min, with a final extension step at 74 °C for 10 min. PCR products were visualized by ethidium bromide agarose (Analytical Grade, Promega Co., Annandale, New South Wales, Australia) gel electrophoresis. A 1 Kb DNA ladder (Invitrogen, Mulgrave, Victoria, Australia) was used to determine the PCR product lengths. PCR products were ligated into pGEM-T Vector System (Promega) according to the manufacturer's instructions. Transformation was carried out using Top 10 Competent Cells (Invitrogen) as per the manufacturer's instructions and grown on LB with ampicillin for blue-white colony screening. Clones containing inserts were subjected to PCR amplification using SP6 and T7 vector primers and the generated amplicons were subjected to restriction fragment length polymorphism analysis to determine operational taxonomic units. Individual PCR products were digested using restriction enzymes MspI and Hin P1 (New England Biolabs Inc., Arundel, Queensland, Australia). The digestion mixture contained 0.05 μl of MspI (20 000 U ml−1), 0.1 μl HinP1 (10 000 U ml−1), 2 μl of 1 × NE Buffer 2 (New England Biolabs) and 17.85 μl of PCR product. The restriction-digested fragments were visualized on 3% Tris-acetate-EDTA high resolution (Promega) agarose gels following 40 V and 200 mA for 2.5 h. Primers used for sequencing specific clone inserts were 917r (Schafer and Muyzer, 2001), 63f and 1387r.
The BLAST (http://www.ncbi.nlm.nih.gov) software tool was utilized to determine closest sequence matches. Sequences were further analyzed by using ARB (http://www.arb-home.de/conf.html) to generate similarity matrices.
Isolation of hydrogen-oxidizing bacteria
Biofilm samples of the open air cathodes at day 83 were taken and cultivated on plates containing per litre 3 g Na2HPO4, 1.5 g. KH2PO4, 0.05 g NH4Cl, 14.7 mg CaCl2, 0.5 g NaCl, 0.247 g MgSO4.7H2O, 1 ml of a trace element solution (Rabaey et al., 2005c), 15 g agar and 1 ml of a vitamin solution (Sigma MEM vitamin solution +20 mg B12). The plates were placed in an anaerobic jar wrapped in aluminum foil. The electron donor and acceptor were provided by branching an electrolysis cell to the anaerobic jar and leading the produced gas (containing approximately 66% H2 and 34% O2) first through a humidifier (containing carbonate buffer) to preclude desiccation and to provide carbon dioxide. Controls were inoculated plates incubated outside the anaerobic jars and uninoculated plates incubated inside the anaerobic jars. The electrolysis cell was operated under a constant current of 2 A, with ruthenium oxide and titanium electrodes (Magneto Anodes, Schiedam, The Netherlands) and 1 N sulfuric acid as electrolyte. This creates a headspace containing only hydrogen and oxygen gas. Colonies were picked from the plates after four days of incubation. After several subcultures, morphologically uniform colonies were obtained through streak plating. The isolates were designated KROX1 to 8. DNA extraction, PCR and restriction fragment length polymorphism for the isolates were carried out as outlined for the clone library. The primers used for PCR and sequencing were 27f and 1492r. All sequencing was done at the Australian Genome Research Facility.
Growth of isolates in MFC
Six MFCs were constructed by creating six closed chamber cathodes around a common anode (Figure 1b). The anode was the same as in the mixed culture system, receiving modified M9 medium containing 0.3 g sodium acetate per liter. Each cathode compartment had a volume of 96 ml and contained a carbon fiber cathode (Fiberglast) clamped against the membrane with a stainless steel mesh current collector (Locker). The projected surface area was 48 cm2 in this case. The current collector also provided the contact with the external electrical circuitry. A volume of 30 ml of medium (the same as used for the isolation but without agar and carbonate) was dispensed to the bottom of the chamber. Aeration was achieved by pumping air through an autoclaved glass syringe filled with cotton wool, connected at the bottom of the cathode compartments. All cathodes were individually connected to the anode over a 50 Ω resistor. The MFCs were operated in duplicate for 7 days, during which time the power production capacity was regularly evaluated using polarization curves (1 mV s−1 scan rate). The isolate repetitions were always run in different experiment groups (with other anodes) to exclude the variability between the experiments caused by, for example, differences in anode potential. Sub-samples were taken daily to assess the pH in the cathode compartments, and pH corrections to 6.5 were applied by injecting an appropriate amount of 1 N HCl.
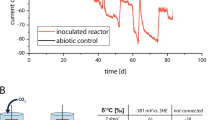
Schematic drawings of the reactor types used. (a) Set-up during the period when anode effluent was used to replenish the cathode liquid. Influent enters the anodic recirculation loop, and acetate is oxidized in the anode. A part of the protons migrate through the cation exchange membrane (CEM) to the cathode, where oxygen is reduced by bacteria growing on the cathode surface. A part of the recirculatory fluid is brought to the cathode, as indicated by the arrow on top. The dotted arrow at the bottom indicates that cathode effluent could also be used to recirculate the anode (Freguia et al., 2007a, 2007b). (b) Top view of the reactor used to test isolates as catalysts for the cathode. Six cathode compartments were connected to a common anode as electron supply. Each cathode was connected to the anode over a separate resistor but no liquid transfer was employed between the anode and the cathode in this case. (1) Cation exchange membranes; (2) cathodic electrodes and (3) air inlet for cathode. In a full loop concept, the cathode and anode liquids can be combined for the recirculation, as indicated by the dotted line.
Results and discussion
The MFC cathodes enabled improved power generation over time
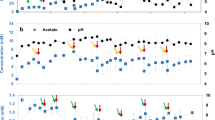
After start-up and inoculation, the initial voltage generated over both R1 (carbon fiber) and R2 (graphite felt) was below 20 mV, despite a rapid decrease of the anode potential to below −200 mV versus SHE. This demonstrates the large overpotential existing for oxygen reduction at non-catalyzed materials (Figure 2), supporting previous observations (Zhao et al., 2006). Reducing this potential loss can be achieved by either supplying catalysts or by drastically increasing the surface area of the cathode (Freguia et al., 2007a, 2007b). Over a period of 48 days, the power output increased, reaching maximal currents of 0.4±0.2 and 0.5±0.2 A m−2 for the R1 and R2, respectively (all current densities are normalized per projected cathode surface) (1.3±0.3 and 2.1±0.3 W m−3 reactor, respectively). A constant increase of the pH in the cathodic compartment occurred owing to proton consumption as oxygen was reduced. The accumulation of breakdown products plus this increase in pH was possibly one of the factors limiting the attainable current and regular decreases of performance observed. This corroborated earlier findings with Nafion (Rozendal et al., 2006) that protons migrate insufficiently through cation exchange membranes at pH values above 3–4. An accidental failure of the recirculation caused an apparent inactivation of attached bacteria, leading to a gradual failure of both reactors, with potentials dropping from above 175 to below 5 mV. Most notably, after replenishment with water, the biocathodes resumed their original potential only after about 5 h, which is quite fast for a biological reaction (very likely indicating only inhibition rather than toxic effects) but considerably slower than would be expected if the cathodes were only chemically catalyzed. The effluent originating from the anode was from day 48 onward used as influent for the cathode. This partially alleviates the need for protons to migrate through the proton exchange membrane, as it has been shown that other cations are capable of fulfilling the role of positive charge transporters (Rozendal et al., 2006). This caused a substantial increase of the current to 1.2±0.6 and 1.1±0.5 A m−2 for the carbon fiber and the graphite felt cathode, respectively, with maximum values (hour averaged) of 2.8 and 2.6 A m−2, respectively (66 and 57 W m−3 reactor, respectively). This is on the same level as the currents previously reported (Clauwaert et al., 2007b), being 0.9–2.7 A m−2 for a tubular MFC (calculated values). The current averages and maximal values are also comparable to those achieved with platinum catalyzed cathodes (Cheng et al., 2006) or pyrolyzed iron(II) phthalocyanine catalyzed electrodes (Zhao et al., 2006) used in conjunction with MFCs. Bearing in mind the longevity (over two months in this study) and self-regenerating capacity of the bacterial process, this offers an attractive alternative to the chemically catalyzed cathodes.
Polarization curves (0.2 mV s−1, 120 min OCV before run, forward sweeps) for the reactors using a mixed population (R2) to biocatalyze the MFC cathode. (a) Power output (mW m−2 cathode projected surface) in function of current (A m−2 cathode projected surface). (b) MFC voltage in function of the current (A m−2 cathode projected surface). The three curves represent the performance at the end of period 2 (a) (day 83), at the end of period 3 (b) (day 212) and at startup (c). MFC, microbial fuel cells; OCV, open circuit voltage.
In the third phase (after 83 days), the graphite felt of R2 was removed and two identical reactors were created by dividing the R1 carbon fiber (minus one third of the electrode) over two reactors and completing the cathodes by adding new carbon fiber. The other third of the R1 carbon fiber mat was used for further microbial analysis. This intervention quite rapidly caused a decrease in the performance versus the previous period, with currents averaging 0.6±0.4 A m−2 and 0.7±0.5 A m−2 for both reactors (3.0±1.3 and 4.1±2.1 W m−3 reactor, respectively). The reason for the decrease of both reactors is very likely the carryover of residual acetate from the anode compartment to the cathode compartment during startup in this third phase, as the anode was still supplied with 198 mg acetate per day, whereas the observed current levels only allowed for 68–79 mg acetate removal per day. This caused increased oxygen usage for acetate consumption and decreased oxygen availability for the electrode-dependent community. Once the cathode decreases in electron acceptance capacity, the anode becomes unable to discharge the electrons and activity decreases also in that compartment. The result for our reactors was a decline in performance, as evidenced by the polarization curve recorded during that period (Figure 2, curves b).
Clone library
The microbial community of the cathode of R1 was sampled and analyzed using a 16S rRNA gene-based clone library approach (Table 1). Restriction fragment length polymorphism analysis of 76 clones placed these into six operational taxonomic units. Following sequence analysis of representatives of the operational taxonomic units, most sequences were affiliated with either the Bacteroidetes or the Proteobacteria. Such microbial diversity over several phyla has previously been described for anode reducing communities (Logan and Regan, 2006). In a related study that examined the microbial community composition of a nitrate reducing biocathode, a similar breadth of phylogenetic diversity was detected (β and γ Proteobacteria, Bacteroidetes) (Virdis and co-workers, unpublished results). The capacity to use a cathode appears widespread among bacteria, and the similarities between anode and cathode reducing/oxdizing populations may indicate the capability of many organisms to perform electron transfer both to and from electrodes, as has been shown for G. metallireducens (Gregory et al., 2004).
The dominant sequence (30% of the clones) in the clone library was most closely related to a Sphingobacterium multivorum strain (also sequenced as AB100738). Other significant sequences were closely related to Bacteroides uncultured clones LYH (EF188284) and PHOS-HE28 (AF314421) (12% and 10% of clones, respectively) to Acinetobacter baumannii (5% of clones), A. calcoaceticus (5% of clones), Acidovorax sp. (3% of clones) and Sphingomonas sp. (3% of clones).
Cathode utilizing organisms can switch to hydrogen as electron donor
After 5 days of growth on plate and with a hydrogen/oxygen atmosphere, several colonies were selected and purified through repetitive transfer. On control plates that were incubated in ambient air, no growth was detected, indicating the need for hydrogen gas as electron donor. Eight isolates, designated KROX1 to KROX8, were further analyzed. After analysis of the 16S rRNA genes, KROX2 to 7 were closely related to the S. multivorum strain previously found to be highly identical to the clone sequences (see above, Table 1). Morphological variations were noted between these strains when grown on solid media and some of the isolates demonstrated swarming behavior, whereas others formed clear, pinpoint colonies. However, morphology is known to be a poor characteristic when attempting to demonstrate strain variation. Isolate KROX1 was highly identical to A. calcoaceticus, whereas KROX8 was identified as a β-Proteobacteria uncultured clone.
Isolates can catalyze oxygen reduction in pure culture MFCs
A series of MFCs (Figure 1b) were operated in which pure culture bacteria were cultivated in separate aerated compartments. For a number of isolates, a significant increase of the current versus the control reactors was noted (Table 2). However, the current could not be maintained for prolonged time periods. The generation of current caused an increase of the pH in the cathode compartment. This was very likely a result of imperfect proton migration through the membrane and the utilization of protons during oxygen reduction. For the biocatalyzed cathodes, this pH increase occurred more rapidly at values of 9.5 and higher. This caused a faster decay of the current versus the non-inoculated control, which had a low current during operation and therefore slow pH increase. This problem was less noted for the mixed population parent MFC, as it was operated in a loop concept that replenished the cathode with protons originating from the anode, aside from acetate at low concentrations and possibly secondary metabolites produced by the anode bacteria. Better control strategies will be needed to fully elucidate the current generating potential of the isolates.
Bacteria reduce the cathodic overpotential losses and hence facilitate oxygen reduction
Owing to rapid consumption, the availability of organic electron donors is often limited in aerobic oligotrophic environments. Thermodynamically, the cathode of an MFC is an attractive electron donor in most cases and it provides a continual supply of electrons. However, activation losses between the cathode and the bacterial electron transport system (possibly evidenced by the potential drop at low current densities in curve b, Figure 2b) decrease the available potential difference for energy generation. The necessity to develop structures or produce soluble redox active metabolites that allow extracellular electron transfer requires additional energy investments. Despite these hurdles, both the mixed population and the pure culture bacteria appear to strongly decrease the overall overpotential for oxygen reduction at the cathode. Scanning electron micrographs of carbon fibers from the cathode showed sparse coverage of bacteria (Figure 3). The perpendicular positioning of bacteria on the fiber surface suggested unfavorable attachment conditions on the smooth and non-functionalized carbon matrix, which could have resulted in limited cathode reactivity. Aelterman et al., recently described that anodophiles as having an activity of up to 659 mA per gram biomass-carbon. If a comparable bacterial activity can be expected at the cathode, only about 66 mg biomass-carbon could be required to explain the activity in our system. Surface modifications such as carboxylation likewise to the anode modifications (Liu et al., 2007) could increase the reactivity and enable an improved colonization on the cathode.
Scanning electron micrographs of the carbon fibers obtained from the cathode. (a) Several carbon fibers, showing dispersed bacteria and small precipitates onto an overall smooth surface. (b) Close-up of a single bacterium standing almost perpendicularly on the fiber surface. Overall, the bacterial densities on the fibers were exceptionally low, with most bacteria standing perpendicularly on the surface. Confocal laser scanning microscopy confirmed that this perpendicular positioning was not an artefact of the SEM method (results not shown).
Isolates are unable to reach the power densities observed for a mixed population
Obtained isolates were unable to reach the power and current levels demonstrated by the mixed population (Figure 4). Aside from the common observation that thus far microbial communities in the anode have far outperformed pure culture MFCs when the same reactor configuration was used, there are several reasons related to the mode of operation that may contribute to this. First, the pH could not be controlled in the pure culture studies as well as in the mixed population reactor, where the reuse of anodic effluent helped to stabilize the pH. Second, in these systems, the bacterial densities could not be verified—which implies that the mixed population could have evolved to higher densities—and hence carry more potential for the current generation. Third, the reuse of anodic effluent in the mixed population reactor inevitably caused a small transfer of organic carbon (acetate and metabolites) to the cathode. Perhaps the organisms at the cathode are lithoheterotrophic, as previously described for denitrifying iron oxidizing organisms (Benz et al., 1998). If so, the organisms used the available organics as carbon source for growth, whereas the cathode was used as source of reducing power for the energy metabolism. In a previous study, (Gregory et al., 2004), Geobacter sulfurreducens needed to be supplied with acetate at start-up; otherwise, the reduction of fumarate could not be established. Whether the bacteria stopped growing after depletion of the acetate was not established. This type of metabolism would give the organisms maximized growth potential. Upon increasing acetate concentrations, this metabolism would fail owing to increased oxygen consumption by conventional heterotrophy. Further research is warranted to elucidate whether lithoheterotrophy is the active metabolism and whether regular spiking of organics as a carbon source can enable alternating heterotrophic/autotrophic growth.
Polarization curves (1 mV s−1, 120 min in OCV before run) for a MFC using isolate 5 as the cathode biocatalyst. (a) Power (mW m−2 CPS) as a function of the current flowing through the MFC. (b) MFC voltage (V) and anode and cathode potential versus SHE (V). Clearly, the main losses can be found at the cathode side of the MFC, as the cathode potential diminishes far more than the anode potential in function of the current (cathode potential measurement can be slightly offset due to the fact that the reference electrode was inserted in the anode). This observation was consistent for all isolates. CPS, cathode projected surface; MFC, microbial fuel cells; OCV, open circuit voltage; SHE, standard hydrogen electrode.
Cathodes versus electron donors in the environment
There are several peculiarities relating to the cathodic electron transfer described here. First, oxygen generally competes for the oxidation of inorganic electron donors such as ferrous iron or sulfides. This makes the electron donor no longer available to the microorganisms (Rentz et al., 2007). This effect was occasionally present in the cathodic electron transfer, as the overpotential for direct reduction of oxygen at carbon electrodes is high (Zhao et al., 2006). This was also demonstrated by the low current generation by a non-colonized cathode at the MFC start-up. Therefore, even in fully saturated oxygen conditions, the electron donor remained available for bacteria as there appear to be hardly any chemical processes competing with the biological ones. Second, extracellular electron transfer is required for the uptake of electrons. The analog in the natural environment appears to be the oxidation of macromolecular or colloidal humics, which cannot enter the cell, by bacteria that use oxygen as an electron acceptor. The fact that humics have been reported to retain reducing capacity even in oxic conditions (van Trump et al., 2006) would support this possibility.
Conclusions
The capacity to use cathodes from MFC as electron donors appears widespread in the microbial world. Here, we demonstrated that bacteria efficiently link the oxidation of a cathode to the reduction of oxygen. Although pure cultures of bacteria could already achieve a significant improvement, we were unable to reach the levels attained by a mixed population comprising mainly Proteobacteria and Bacteroidetes species. Technical hurdles, such as pH control and surface modifications, need to be overcome to fully exploit this bacterial catalytic capacity. If the energy investment to maintain suitable biofilm growth conditions at the cathode is kept low, bacteria may well be the most sustainable long-term catalysts for cathodic oxygen reduction.
References
Aelterman P, Freguia S, Keller J, Verstraete W, Rabaey K . (2008). The anode potential regulates bacterial activity in microbial fuel cells. Appl Microbiol Biotechnol; e-pub ahead of print (doi:10.1007/s00253-007-1327-8).
Aelterman P, Rabaey K, The Pham H, Boon N, Verstraete W . (2006). Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ Sci Technol 40: 3388–3394.
Benz M, Brune A, Schink B . (1998). Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemohetorotrophic nitrate-reducing bacteria. Arch Microbiol 169: 159–165.
Bergel A, Feron D, Mollica A . (2005). Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochem Commun 7: 900–904.
Bond DR, Lovley DR . (2003). Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69: 1548–1555.
Cheng S, Liu H, Logan BE . (2006). Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem Commun 8: 489–494.
Clauwaert P, Rabaey K, Aelterman P, De Schamphelaire L, The Pham H, Boeckx P et al. (2007a). Biological denitrification driven by microbial fuel cells. Environ Sci Technol 41: 3354–3360.
Clauwaert P, van der Ha D, Boon N, Verbeken K, Verhaege M, Rabaey K et al. (2007b). An open air biocathode enables effective electricity generation with microbial fuel cells. Environ Sci Technol 41: 7564–7569.
Freguia S, Rabaey K, Yuan Z, Keller J . (2007a). Sequential anode-cathode configuration improves cathodic oxygen reduction and effluent quality of microbial fuel cells. Water Res In Press, Corrected Proof, Available online 11 October 2007.
Freguia S, Rabaey K, Yuan Z, Keller J . (2007b). Non-catalyzed cathodic oxygen reduction at graphite granules in microbial fuel cells. Electrochimica Acta 53: 598–603.
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A et al. (2006). Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA 103: 11358–11363.
Gregory KB, Bond DR, Lovley DR . (2004). Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6: 596–604.
He Z, Angenent LT . (2006). Application of bacterial biocathodes in microbial fuel cells. Electroanalysis 18: 2009–2015.
Liu H, Ramnarayanan R, Logan BE . (2004). Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38: 2281–2285.
Liu JL, Lowy DA, Baumann RG, Tender LM . (2007). Influence of anode pretreatment on its microbial colonization. J Appl Microbiol 102: 177–183.
Logan BE, Regan JM . (2006). Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14: 512–518.
Logan B, Aelterman P, Hamelers B, Rozendal R, Schroder U, Keller J et al. (2006). Microbial fuel cells: methodology and technology. Environ Sci Technol 40: 5181–5192.
Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ et al. (1998). Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA (64: 795, 1998). Appl Environ Microbiol 64: 2333.
Rabaey K, Verstraete W . (2005). Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol 23: 291–298.
Rabaey K, Boon N, Höfte M, Verstraete W . (2005a). Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol 39: 3401–3408.
Rabaey K, Clauwaert P, Aelterman P, Verstraete W . (2005b). Tubular microbial fuel cells for efficient electricity generation. Environ Sci Technol 39: 8077–8082.
Rabaey K, Ossieur W, Verhaege M, Verstraete W . (2005c). Continuous microbial fuel cells convert carbohydrates to electricity. Water Sci Technol 52: 515–523.
Rabaey K, Van de Sompel K, Maignien L, Boon N, Aelterman P, Clauwaert P et al. (2006). Microbial fuel cells for sulfide removal. Environ Sci Technol 40: 5218–5224.
Rabaey K, Rodriguez J, Blackall LL, Keller J, Gross P, Batstone D et al. (2007). Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J 1: 9–18.
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR . (2005). Extracellular electron transfer via microbial nanowires. Nature 435: 1098–1101.
Rentz JA, Kraiya C, Luther GW, Emerson D . (2007). Control of ferrous iron oxidation within circumneutral microbial iron mats by cellular activity and autocatalysis. Environ Sci Technol 41: 6084–6089.
Rozendal RA, Hamelers HVM, Buisman CJN . (2006). Effects of membrane cation transport on pH and microbial fuel cell performance. Environ Sci Technol 40: 5206–5211.
Schafer H, Muyzer G . (2001). Denaturing gradient gel electrophoresis in marine ecology. Methods Microbiol 30: 425–468.
Thrash JC, VanTrump JI, Weber KA, Miller E, Achenbach LA, Coates JD . (2007). Electrochemical stimulation of microbial perchlorate reduction. Environ Sci Technol 41: 1740–1746.
van Trump JI, Sun Y, Coates JD . (2006). Microbial interactions with humic substances. In: Laskin AI, Sariaslani S, Gadd GM (eds). Advances in Applied Microbiology. Elsevier: San Diego, pp 55–96.
Zhao F, Harnisch F, Schroder U, Scholz F, Bogdanoff P, Herrmann I . (2006). Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ Sci Technol 40: 5193–5199.
Acknowledgements
We acknowledge Romain Lemaire for his help with electron microscopy. Korneel Rabaey is supported by the University of Queensland Postdoctoral Research Fellow Scheme, the UQ Early Career Researcher Scheme and the Australian Research Council—Discovery Program (DP0666927). Peter Clauwaert is supported by a PhD grant (IWT Grant 53305) of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabaey, K., Read, S., Clauwaert, P. et al. Cathodic oxygen reduction catalyzed by bacteria in microbial fuel cells. ISME J 2, 519–527 (2008). https://doi.org/10.1038/ismej.2008.1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2008.1
Keywords
This article is cited by
-
Microbial community dynamics in electroactive biofilms across time under different applied anode potentials
Sustainable Environment Research (2022)
-
Trickling filter in a biocathode microbial fuel cell for efficient wastewater treatment and energy production
Science China Technological Sciences (2019)
-
Continuous and scalable applications of microbial fuel cells: a critical review
Reviews in Environmental Science and Bio/Technology (2019)
-
Analysis of long-term performance and microbial community structure in bio-cathode microbial desalination cells
Environmental Science and Pollution Research (2016)