Abstract
Hydrogen is a key intermediate element in microbial electrosynthesis as a mediator of the reduction of carbon dioxide (CO2) into added value compounds. In the present work we aimed at studying the biological production of hydrogen in biocathodes operated at − 1.0 V vs. Ag/AgCl, using a highly comparable technology and CO2 as carbon feedstock. Ten bacterial strains were chosen from genera Rhodobacter, Rhodopseudomonas, Rhodocyclus, Desulfovibrio and Sporomusa, all described as hydrogen producing candidates. Monospecific biofilms were formed on carbon cloth cathodes and hydrogen evolution was constantly monitored using a microsensor. Eight over ten bacteria strains showed electroactivity and H2 production rates increased significantly (two to eightfold) compared to abiotic conditions for two of them (Desulfovibrio paquesii and Desulfovibrio desulfuricans). D. paquesii DSM 16681 exhibited the highest production rate (45.6 ± 18.8 µM min−1) compared to abiotic conditions (5.5 ± 0.6 µM min−1), although specific production rates (per 16S rRNA copy) were similar to those obtained for other strains. This study demonstrated that many microorganisms are suspected to participate in net hydrogen production but inherent differences among strains do occur, which are relevant for future developments of resilient biofilm coated cathodes as a stable hydrogen production platform in microbial electrosynthesis.
Similar content being viewed by others
Introduction
Microbial electrosynthesis (MES) is engineered to use electric power and carbon dioxide (CO2) as the only energy and carbon sources in reductive bioelectrochemical processes for biosynthesis1. Among potential uses of MES, alternative biofuels production copes for most of the scientific attention and deserves an intense research activity2,3. Some microorganisms are able to transfer electrons to or from a poised solid electrode4, a talent that contributed to develop a broad range of practical applications from bioenergy to water treatment5,6. Bioelectrochemical systems (BES) exploit the capacity of these electroactive microorganisms able to capture electrons and transform them into soluble energy containing compounds (organic multicarbon molecules)7. A major limitation in BES processes is the rate at which microorganisms acquire electrons from solid state electrodes for CO2 reduction8. Several studies have proposed hydrogen (H2) as the principal electron donor intermediary in the production of commodity chemicals from carbon dioxide and electricity9,10,11. Molecules such as H2, carbon monoxide (CO) and formate are the most preferable for microbial catalysts12. Microbial electrosynthesis will be reinforced by the integration of proper H2-producing microbial catalysts.
Metabolically, surplus hydrogen production for most anaerobic microorganisms is an induced response in order to avoid accumulation of reduced cofactors (NAD, NADP, FAD, ferredoxins, and others) so that metabolic processes can continue13. Hydrogenases and nitrogenases are among the most widespread enzymes involved in proton reduction for hydrogen production. Hydrogenases are responsible of the reversible reaction to convert protons and electrons into hydrogen (2H+ + 2e− \(\leftrightarrow\) H2). Contrarily, nitrogenases naturally produce H2 as a by-product of nitrogen fixation (N2 + 8e− + 8H+ + 16ATP \(\leftrightarrow\) 2NH3 + H2 + 16ADP + 16 Pi). Under nitrogen limitation, nitrogenases function as a hydrogenase and only produce H2 by proton reduction to molecular hydrogen (2H+ + 2e− + 4ATP \(\leftrightarrow\) H2 + 4ADP + 4Pi)13,14.
In biocathodes, H2 is produced either abiotically (pure electrocatalytic process) or biotically, with the participation of living microorganisms or isolated enzymes. Abiotic or electrocatalytic H2 is produced when using carbon-based materials (i.e. graphite cathodes) at cathode potentials below − 0.8 V vs. Ag/AgCl9. Biologically produced H2 (BioH2) have been proven in biocathodes by using both pure and mixed microbial cultures. Geobacter sulfurreducens, Rhodobacter capsulatus, and Desulfovibrio spp. catalyze hydrogen production at cathode potentials below − 0.8 V vs. Ag/AgCl10,15,16,17. Microbial community characterizations demonstrated that highly H2 producing biocathodes were enriched mainly by Proteobacteria10,11,18. Croese and co-workers described a cathodic microbial community mainly composed of Deltaproteobacteria in which Desulfovibrio spp. were the most abundant18. Alpha- and Betaproteobacteria (Rhodocyclaceae) have also been highlighted to be mediating H2 production in cathodes10,11. In addition, increased H2 production rates in the presence of cell-free exhausted medium from cultures of Sporomusa sphaerodies, Sporomusa ovata and Methanococcus maripaludis have also been confirmed19,20,21. The authors demonstrated that the former presence of microorganisms in the reactor had changed the electrode surface via metal deposition (nickel and cobalt) leading to an increased H2 production19. Alternatively, free enzymes (hydrogenases and formate dehydrogenases) previously released by microorganisms20,21 could also be deposited in the electrode surface reinforcing H2 production yields.
Another feasible strategy aiming to improve H2 production in biocathodes is the integration of low-cost metal-based cathode materials such as cobalt phosphide, molybdenum disulfide and nickel-molybdenum with putatively electroactive microorganisms. Although its integration with the required conditions for microbial growth might cause toxicity towards microorganisms22, some of these materials have been demonstrated as a promising and biocompatible electrocatalytic H2-producing platform, while combining with CO2-reducing and H2-utilizing bacteria (like S. ovata and M. maripaludis) ensuring higher value-added chemicals production23.
In the present work we aimed at assessing the capacity of ten strains of Rhodobacter, Rhodopseudomonas, Rhodocyclus, Desulfovibrio and Sporomusa previously reported as potential cathodic H2 producers. This approach may allow selecting potential candidates to stablish resilient co-cultures, ideally composed of a H2 producing strain in combination with a homoacetogen, for microbial electrosynthesis processes and electro-fermentation. We used an optimized experimental protocol which could facilitate the comparison among strains thus limiting the effect of heterogenous reactor designs and analytical tools which are found in the literature. H2 evolution was continuously monitored by means of an H2 microsensor placed directly in contact with liquid medium and close to cathode surface. H2 production efficiencies in monospecific biofilms of each strain were analyzed repeatedly and compared to abiotic conditions.
Results and discussion
Electrocatalytic H2 production at carbon cloth electrodes
Accurate choice of control conditions in reactor set-ups (as abiotic controls) is mandatory to avoid data deviation and facilitate interpretation20. Several tests were carried out to determine the electrocatalytic (or abiotic) H2 production in carbon cloth electrodes. The use of a fixed methodology and an exhaustive analysis of control experiments facilitated comparison among strains and detection of relevant biotic effects. Carbon cloth electrodes were operated at different potentials (− 0.6, − 0.8 and − 1.0 V vs. Ag/AgCl) and H2 evolution was monitored. Under these conditions, catalytic H2 was only detected when cathodes were poised at − 0.8 and − 1.0 V vs. Ag/AgCl (Supplementary Table S1). Independently of the medium used, higher H2 production rates (8.4 ± 3.0, 6.4 ± 1.8 and 5.1 ± 0.8 µM min−1, respectively) were achieved at − 1.0 V.
Electrocatalytic H2 production is conditioned by several factors, such as liquid medium composition, temperature, overpressure, electrode material, reactor designs and/or operating modes22,23,24,25. To estimate if medium composition was affecting hydrogen production, ionic losses were calculated (Supplementary Table S2). DSM 311 and Aulenta et al. modified media had similar ionic losses (+ 85 and + 89 mV, respectively). Modified DSM 27 had slightly higher ionic loss (+ 184 mV) compared to the other media. These differences were related to the different salinities, however observed differences should not have a significant effect on catalytic hydrogen productions.
Despite special care was applied to minimize effects in cathode sizes and qualities, differences in H2 production rates were detected between carbon cloth electrodes, suggesting that parameters such as material integrity or the presence of impurities, could be affecting H2 production. Consequently, it was necessary to measure abiotic H2 concentrations for each carbon cloth electrode later used for monospecific biofilm formation to ensure proper results interpretation.
Formation of monospecific biofilms and stability
Abundance of the 16S rRNA gene was used as a proxy for estimating bacterial density in BES. Although 16S rRNA gene copies could be translated into cell abundance in view of 16S rRNA copies per unit genome26, no such transformation was performed since differences were only analyzed in terms of biofilm stability of individual strains during experiments. As expected, the presence of bacteria from the beginning of the BES operation was confirmed. 16S rRNA gene copies ranged from 3.7 × 106 to 4.5 × 105 gene copies/cm2 in biofilm samples, and from 1.5 × 108 to 9.7 × 104 gene copies/mL in bulk liquid.
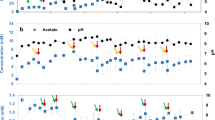
Proportions between abundances measured before and after BES operation were used to assess the short-term stability of the biofilm. Except for Rhodopseudomonas pseudopalustris DSM 123 and isolate C2T108.3, all bacterial strains tended to remain attached into the cathode during the operation, or even grow as a biofilm, since an increase in gene copies concentrations was found (more than tenfold increase for Rhodobacter capsulatus DSM 152; Fig. 1). Total 16S rRNA gene copies in BES (i.e. bulk liquid + biofilm cells) remained almost invariable during operation, except for strains DSM123 and C2T108.3 which experienced a significant decrease, confirming no growth occurred. Collectively, abundance data indicates that monospecific biofilms could be effectively formed and maintained stable for the duration of the experiment.
Bioelectrochemical production of H2 in purple non-sulfur (PNS) bacteria
Inoculation and growth experimental procedure was optimized to enable monospecific biofilm formation on the cathode surface and short-term evaluation of H2 production. As stated above, pure electrocatalytic H2 production was estimated for every cathode and used as a threshold to determine the effect of the bacterial presence.
For all tested PNS strains, H2 production started immediately after feeding reactors with CO2, and accounted for higher productions (1.2 to 2.2-fold) compared to abiotic conditions (Fig. 2). Specific net productivities (per unit biomass) ranged from 3.6 to 283.3 (µM min−1 × 107 copy−1 16S rRNA). In addition, higher current demands and lower energy consumptions were recorded (Table 1). Strain-specific differences were observed after the two CO2 feeding performed. In particular, H2 production rates for the two Rhodobacter sp. (DSM 5864 and DSM 152) decreased to values similar to abiotic conditions (6.7 ± 0.6 and 5.9 ± 1.8 µM min−1, respectively). In contrast, for Rhodopseudomonas sp. (DSM 123 and isolates C2T108.3 and C1S119.2) and Rhodocyclus tenuis biofilms, successive CO2 feedings caused a severe decrease of H2 production rates (from two to sevenfold compared to maximum production). Differences in current demand and energy consumption occurred accordingly (Table 1). Decreasing H2 production rates with R. pseudopalustris DSM 123 and isolate C2T108.3 could be explained due to the large decrease (from 1 to 3 magnitude orders) observed in cell densities attached to the cathode over experimental time (Fig. 1). Coulombic efficiencies (CE) for the production of H2 remained at similar values to those found in abiotic condition when using the two Rhodobacter sp., except after the second CO2 feeding for DSM 5864 (CE = 67.8%). Also, when testing R. tenuis DSM 112 CE remained similar to abiotic conditions after the second CO2 feeding (76.4% and 83.5%, respectively). Contrarily, coulombic efficiencies were severely lower when using Rhodopseudomonas sp. in comparison with abiotic conditions (Table 1).
Hydrogen production rates (µM min−1) of monospecific biofilms of purple non sulfur (PNS) bacteria after successive CO2 feeding in BES reactors. Rates are compared to values obtained for the same electrode in abiotic conditions (white bar) and percentages indicate production rates increase or decrease (negative values).
pH has a great impact on electrochemical performance. According to the Nernst equation and typical solution conditions, an overpotential of − 59 mV is expected per pH unit increase27. For that, pH was measured several times during BES operation (Supplementary Table S3). For all the tested bacteria starting pH was between 6.8–7.0. pH plummeted to 5.4–5.7 immediately after sparging pure CO2 for over 10 min and increase continuously afterwards. From thermodynamic point of view, such acidic pH reinforced the production of hydrogen. During the experimental period pH followed different trends for each strain. pH decreased to around 5.7–5.8 in the two Rhodobacter sp. (DSM 5864 and DSM 152), while for isolate C1S119.1 went up to 7.4 after five days of operation. Similar pHs were measured for the other PNS strains (around 6.6–6.9 at the end of operation). Differences were linked to the rate of bioelectrochemical H2 production of each strain and the buffer capacity of each medium (Supplementary Table S4).
Hydrogen production by PNS occurs under photoheterotrophic metabolism14. Although several studies have highlighted the metabolic versatility of PNS, focusing mainly in the in-situ bioH2 production under different substrates, reactor designs and environmental conditions13,14,28,29, few studies have been conducted using pure cultures in BES10,30,31. Observed changes in H2 production rates after different CO2 flushes in the reactor when operated with PNS could be due to two opposite effects. First, the possibility that H2 was being produced as a residual activity from photo-fermentative growth. This could explain the higher production rates when starting BES operation, but only if some intracellular carbon reservoir had been accumulated during preparation of biofilms. Both Rhodobacter and Rhodopseudomonas species are able to accumulate polyhydroxy butyrate (PHB) under nutrient starvation (i.e. nitrogen, phosphorus, sulfur) and excess of organic carbon source. Larger amounts of PHB are accumulated under nitrogen limitation and especially when acetate is used as carbon source. These are also the suitable conditions to H2 production via photo-fermentation. On the event of a exposure of cells to non-optimal conditions, PHB can be mobilized and used as energy and carbon source32,33. Second, an enhanced H2 consumption after completed adaptation of cells to the new reactor environment. Although Rhodobacter sp. and Rhodopseudomonas sp. have been proposed as H2-producing bacteria in biocathodes by other authors10,30, in the tested conditions these strains did not show such a capacity in the long-term, i.e. H2 consumption surpassed production after CO2 replenishment. However, current demands increased in three of the tested PNS strains (R. pseudopalustris DSM 123; isolate C2T108.3 and R. tenuis DSM 112) after the second CO2 feeding (Table 1). This may be indicative of H2 being produced and rapidly consumed by the cells. Unfortunately, due to technical limitations for sampling the gas phase, no data for CO2 consumption is available to confirm this hypothesis. An alternative would be a direct electron transfer without H2 accumulation. In this sense, Bose and co-workers tested Rhodopseudomonas palustris strain TIE-1 in cathodes poised at + 0.1 V vs. SHE (− 0.1 V vs. Ag/AgCl) confirming this strain was able to accept electrons from the poised electrode34. Derived electrons from the cathode surface were entering the photosynthetic electron transport chain, leading to a highly reduced cellular environment. Further transcriptomic analysis on the expression levels of ruBisCO forms I and II using wild-type strain and a ruBisCO double mutant, determined that electron uptake was connected to CO2 fixation31.
Bioelectrochemical production of H2 in Sporomusa ovata
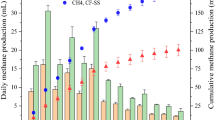
For the system inoculated with S. ovata DSM 2662, H2 production rate was fourfold lower after the first CO2 feeding. Remarkably, after the second feeding no H2 production could be detected (Fig. 3a). Coulombic efficiency for the production of H2 was lower in biotic than in abiotic conditions (46.1% and 80.7%, respectively). Volatile fatty acids and alcohols were measured during BES operation in all the experimental conditions and tested strains, however only when using S. ovata acetate was detected. Acetate concentrations increased over the time reaching 6.4 mM at the end of the operation. Coulombic efficiencies for acetate production increased over operation from 57.2% to 70.3%. Mainly due to acetate production, pH decreased from 7.4 to 6.5 during operation. Sporomusa spp. have been widely used in MES20,35,36, taking advantage of its homoacetogenic metabolism. Therefore, recorded decreasing H2 concentrations in our system could be explained by a quick consumption of H2 for acetate biosynthesis. Deutzmann and co-workers found higher H2 productions when using cell-free exhausted medium from Sporomusa sphaeroides cultures, probably due to the presence of enzymes (such as hydrogenases) into the culture supernatants20. More recently, high H2 evolving BES at different cathode potentials (from − 0.5 to − 0.9 V vs. Ag/AgCl) have been demonstrated by using S. ovata cell-free medium due to nickel and cobalt deposition onto the electrode surface19. Despite being a frequently studied candidate for bioelectrosynthesis development, and considering our main objective, Sporomusa strains seem not to be good candidates for a stable H2 producing platform using modified biocathodes. It seems clear that net H2 production is only observed in the absence of active microorganisms.
Hydrogen production rates (µM min−1) using a) S. ovata DSM 2662 and b) Desulfovibrio strains with poised electrodes at − 1.0 V (upper plots) and − 0.8 V vs. Ag/AgCl (lower plots). Rates are compared to values obtained for the same electrode in abiotic conditions (white bar) and percentages indicate increase or decrease (negative values) in production rates. Statistically significant differences are shown as * (p < 0.05).
Bioelectrochemical production of H2 in sulphate-reducing bacteria
Sulphate-reducing bacteria can use H2 as electron donor while reducing sulphate in anaerobic conditions37. But some of them, specially Desulfovibrio spp., have been postulated as potentially H2 producing microorganisms in BES using pure and mixed cultures11,16,37. Here, three different Desulfovibrio species were selected as potential candidates to test and compare this process with other phylogenetically distinct bacteria. H2 production rates at − 1 V (Fig. 3b) were consistent over time for the three studied species, in agreement with Aulenta and co-workers with D. paquesii DSM 1668116. H2 production rates in D. desulfuricans remained significantly higher compared to abiotic conditions (p ≤ 0.01) after successive CO2 feeding (13 ± 2.9 and 5.7 ± 1.0 µM min−1, respectively; Table 1), but a decrease in production rate was observed. While energy consumptions remained similar between feedings (1.3 × 10−5 to 1.6 × 10−5 kWh), higher current demands were recorded in comparison to abiotic conditions. Coulombic efficiencies for the production of H2 remained above 70% being lower than the abiotic (Table 1). At the same potential, D. vulgaris did not increase production rates when compared to abiotic conditions (3.3 ± 0.9 and 3.2 ± 0.4 – 4.1 ± 0.6 µM min−1, respectively) although a higher current demand was measured (Table 1). The highest increase in H2 production was observed for D. paquesii after two CO2 flushes in the system operated at − 1 V. A significant eightfold increase (p = 0.02) in H2 production rate compared to abiotic conditions (5.5 ± 0.6 and 45.6 ± 18.8 µM min−1, respectively) was observed, leading to an increased current demand but a slightly lower energy consumption. Similarly as exposed by Aulenta and co-workers, coulombic efficiencies (Table 1) remained between 80–100%16. For all the tested Desulfovibrio starting pH was 7.7 and during BES operation pH decreased to 6.2–7.2 (Supplementary Table S3).
Since two out of the three Desulfovibrio strains showed the highest capacity in net H2 production among the ten tested strains, experiments at less reducing potentials (− 0.8 and − 0.6 V vs. Ag/AgCl; Fig. 3) were also performed. When biocathodes were poised at − 0.8 V, measured current demands increased from abiotic to biotic conditions with all Desulfovibrio strains (Table 1). Similar H2 production rates were found for D. desulfuricans and D. vulgaris, being slightly lower than in abiotic conditions. Otherwise, in the presence of D. paquesii DSM 16681, higher net H2 production rate was obtained. H2 production was not detected neither in abiotic nor biotic conditions at − 0.6 V vs. Ag/AgCl. Biocathodes in the presence of D. paquesii DSM 16681 had been characterized before at − 0.9 and − 0.7 V vs. Ag/AgCl by Aulenta and co-workers16. The authors hypothesized that Desulfovibrio could not use efficiently the electrons when cathodes were polarized at potentials above − 0.9 V because the recorded current demands in these conditions were very similar at the ones obtained in abiotic conditions. In contrast, our results suggested that, at least at − 0.8 V vs. Ag/AgCl, D. paquesii was able to produce H2 at higher rates and, with regard to the higher current demand, usage of electrons from the cathode was confirmed.
The obtained results demonstrated that at least D. desulfuricans and D. paquesii were able to increase H2 production in biocathodes at the used experimental conditions. D. paquesii was reported before as an electroactive microorganism able to produce H2 highly efficiently with production rates from five to tenfold times higher than in abiotic conditions, and coulombic efficiencies near 100%16. Also, D. caledoniensis has been proved as a H2 producing catalyst at − 0.8 V vs. Ag/AgCl17. Conversely, in the tested conditions no conclusive results could be obtained for D. vulgaris. However, direct evidence on electrocatalytic H2 production when using purified D. vulgaris [Fe] hydrogenase was obtained when coating cathode electrodes with this enzyme38. Also, a stable hydrogen production using whole cells of D. vulgaris have been reported37, confirming electroactivity of the microorganism in the presence of methyl viologen and electrodes poised at − 0.7 V vs. Ag/AgCl. Although not tested here, the presence of soluble electron shuttles, such as methyl viologen, may impact the H2 production rate, but of course the use of these compounds will impose additional parameters to be controlled in order to develop stable H2 production platforms for BES.
Electrochemical characterization
Biofilms were characterized electrochemically using cyclic voltammetries (CV) after 5 days of operation and compared to abiotic CVs (Fig. 4). In eight out of the ten tested strains, the current demand of biotic CVs differed significantly from the abiotic control indicating that those biofilms were electroactive. Current demand started to increase around − 0.7 to − 0.8 V vs. Ag/AgCl in abiotic conditions while in the presence of DSM 5864, DSM 152, DSM 123, C1S119.2, and DSM 112 increased current demands were recorded at higher potentials, from − 0.4 to − 0.6 V vs. Ag/AgCl. This was interpreted as an indication that the biofilm catalyzed the reduction reaction of protons (H+) decreasing energy losses that could be associated to the catalytic hydrogen production. Highest current demands were observed for C1S119.2 and DSM 112 reaching 14.0 and 18.3 mA, respectively. Even though, these results could not be linked to a higher net H2 production rates. It should be considered that CV measurements are highly sensitive and can detect small induced changes in the redox state of the cell that may not be sustained in the long run.
A redox pair was identified at − 0.65 V vs. Ag/AgCl for Rhodobacter sp. DSM 5864, R. pseudopalustris DSM 123, isolate C1S119.2 and Desulfovibrio spp., which has been typically observed in H2-producing biocathodes9,10 and related to the D. vulgaris [Fe] hydrogenase activity38. Similar results as the presented with Desulfovibrio species were found by other authors using pure cultures of D. caledoniensis and D. paquesii16,17. Redox peaks could not be clearly identified for the experiments with R. capsulatus DSM 152, R. tenuis DSM 112 and S. ovata. An additional reduction peak was found at − 0.46 V when using D. vulgaris. also found in electrochemical experiments conducted with D. gigas [NiFe] hydrogenases in bulk suspension39. Although electrochemical characterization using CV revealed some electroactivity for almost all the strains, unfortunately these could not be translated into direct H2 production, except for D. desulfuricans DSM 642 and D. paquesii DSM 16681. Defined co-cultures where hydrogenotrophic bacteria could be sustained by an efficient biological hydrogen producer has been highlighted before as an important step forward to improve microbial electrosynthesis40. According to our results, Desulfovibrio species are the best candidates, among all tested strains, to further develop the use of biofilm coated cathodes as a stable H2 production platform in microbial electrosynthesis.
Conclusions
In this study we presented an evaluation of ten different bacteria (including species of Rhodobacter, Rhodopseudomonas, Rhodocyclus, Sporomusa and Desulfovibrio) as a first step in the development of a stable H2-evolving platform for microbial electrosynthesis. All used strains and isolates had been previously proved as effective H2 producers in bioelectrochemical systems by different authors. In this work, we tested all strains using an optimized and identical protocol based on the development of monospecific biofilms, thus facilitating comparisons among them. Cell densities measured by qPCR revealed that cells had the tendency to attach to the electrode surface in BES reactors, independently on their ability to produce H2. Hydrogen production rates increased compared to abiotic conditions in all tested strains except S. ovata DSM 2662. In four of them, R. capsulatus DSM 152, isolate C1S119.2, D. desulfuricans DSM 642, D. paquesii DSM 16681, specific H2 production rates were markedly higher but only on Desulfovibrio strains were sustained in the long term. This fact, together with a major stability of the biofilms of these species, resulted in D. desulfuricans DSM 642 and D. paquesii DSM 16681 as the most promising candidates to evolve selective biologic H2-producing cathodes. Despite differences in production rates, eight strains presented some electroactivity according to cyclic voltammetry measurements and are candidates to additional explorations of their performance in BES under changing conditions. Our results represent a significant step forward to further study H2-producing bacteria into defined co-cultures for microbial electrosynthesis and electro-fermentation.
Materials and methods
Bacterial strains and maintenance conditions
All bacterial strains used in this study were obtained from Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures, except for isolates C2T108.3 and C1S119.2 obtained from a denitrifying biocathode and tentatively identified as Rhodopseudomonas30. Rhodobacter sp. DSM 5864, Rhodobacter capsulatus DSM 152, Rhodopseudomonas pseudopalustris DSM 123, Rhodocyclus tenuis DSM 112 and isolates C2T108.3 and C1S119.2 were cultured using DSM 27 medium. Sporomusa ovata DSM 2662 was routinely cultured on DSM 311 medium and Desulfovibrio paquesii DSM 16681, Desulfovibrio vulgaris DSM 644, Desulfovibrio desulfuricans DSM 642 using DSM 63 medium. All cultures were grown at recommended culturing conditions following the instructions provided by the DSMZ. Culture inoculation and maintenance was done into serum bottles with butyl rubber septa under anaerobic conditions. All manipulations were carried out in an anaerobic chamber (gas mixture N2:H2:CO2 [90:5:5], COY Laboratory Products, INC, USA).
Biofilm development on carbon cloth electrodes
In order to stimulate formation of monospecific biofilms on carbon cloth, slight modifications of culture conditions were applied (Supplementary Tables S5–S7). Inclusion of one source of organic matter was done as an adaptation for autotrophic conditions finally used during BES operation. Modifications of DSM 27 included: substitution of NH4-acetate for Na-acetate (0.53 g L−1) and exclusion of yeast extract, Na-succinate, Na-resazurin solution and NH4Cl. pH was set to 6.8, and medium was degasified with helium (He) to reduce the presence of N2 into the medium. All modifications were carried out to avoid product inhibition of the nitrogenase activity, and force bacteria to get rid of the excess energy and reducing power through H2 production. Modifications of DSM 311 medium included: exclusion of casitone, betaine, L-cysteine-HCl, Na2S and Na-resazurin, solution was set at pH 7 and degasified with nitrogen (N2). Modified medium specially designed was used for Desulfovibrio species16. For all strains and isolates, brand-new treated carbon cloth electrodes were immersed in freshly prepared media modified to enhance bacterial growth and biofilm formation. Incubations were performed in 100 mL serum bottles. Rhodobacter, Rhodopseudomonas (including isolates C2T108.3 and C1S119.2) and Rhodocyclus species were incubated under constant light at 25 °C, while Sporomusa ovata and Desulfovibrio sp. were maintained in the dark and 30 °C. As for bacterial strains maintenance, all manipulations were carried out under anaerobic conditions. Biofilms were allowed to grow on the surface of electrodes for a minimum of 10 days.
Bioelectrochemical system setup and operation
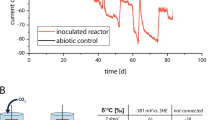
H-type bioelectrochemical systems (BES) reactors with a nominal capacity of 150 mL (Adams & Chittenden Scientific Glass, Berkeley CA – USA) were used. Each one consisted of two chambers, anodic and cathodic, separated by a cation exchange membrane (21.2 cm2 surface area; CMI-7000, Membranes International Inc, USA). Carbon cloth (NuVant’s ELAT LT2400W, FuelCellsEtc USA) with 24 cm2 surface area connected directly to a stainless-steel wire (AISI 304 Grade and 1 mm thickness) was used as cathode electrode (working electrode). The contribution of the wire to H2 production was considered to be negligible. An Ag/AgCl reference electrode (+ 197 mV vs. SHE, sat KCl, SE11 Sensortechnik Meinsberg, Germany) was placed into the cathodic chamber. A graphite rod (5 × 250 mm, MERSEN IBERICA, Spain) was used as anode (counter electrode) (Supplementary Fig. S1). Prior to usage, carbon cloth pieces meant to be used as electrodes were cleaned with 0.5 M HCl, 0.5 M NaOH and miliQ water for 12 h each solution to remove impurities. Chronoamperometric experiments were performed in abiotic and biotic conditions at − 1.0, − 0.8 and − 0.6 V vs. Ag/AgCl using a potentiostat (BioLogic, Model VSP, France). All the potentials indicated in this work are relative to Ag/AgCl. H-type cells were maintained at 30 ± 2 °C, with constant stirring by means of a magnetic bar at 200 rpm (MultiMix D9 P V1, OVAN, Spain) and in the dark.
Anodic and cathodic chambers were filled with the corresponding modified inorganic media (Supplementary Tables S5–S7). Abiotic (cell-free) cathodes poised at − 1.0 V vs. Ag/AgCl were used to test BES set-up. After 5–6 h of operation, H2 saturation was reached (~ 800 µM, in view of the media composition and reactor temperature). Slight increases from saturation value were recorded over time if overpressure was allowed to the system. The presence of leaks in the reactor were tested after disconnecting the potentiostat and recording the H2 concentration decrease. Measured leaks did not exceed 3.5 µM h−1.
After abiotic tests were performed, the same electrode was incubated in the presence of bacteria until a biofilm was formed (see previous subsection). Carbon cloth electrodes with a monospecific biofilm formed on its surface were placed directly into the cathodic chamber. Remaining cells into the supernatants (90 mL) were harvested in the late exponential phase at an optical density (OD600) of 0.3–0.4, pelleted by centrifugation (4,400 rpm, 15 min, 4 °C), resuspended into 1 mL of inorganic modified medium and added into the cathodic chamber. Headspace was saturated with filter-sterilized pure CO2 at the beginning of the operation. Biotic experiments lasted 5 days. Once set-up was completed, H2 production rates were re-evaluated and compared to abiotic tests using the same operational conditions (cathodic voltage − 1.0 V vs. Ag/AgCl) and maintained for 3 days. After this, reactor headspace was flushed with a filter-sterilized pure CO2 stream, and production re-evaluated for two additional days (second CO2 feeding). Flushing was repeated at day 5 to ensure inorganic carbon source availability before cyclic voltammetries were done.
Electrochemical characterization and calculations
On-line hydrogen concentration measurements were performed using a H2 NP-500 microsensor (Unisense, Denmark) directly placed in the liquid compartment close to the cathode surface. Microsensors were regularly calibrated using a saturated water solution using CO2:H2 gas mixture (80:20% v/v) following the specifications of the manufacturer. Liquid samples from the cathodic chamber were taken during biotic operation to control pH and volatile fatty acids (i.e. acetate), and alcohols (i.e. ethanol) concentration. VFA and alcohols were analyzed using a gas chromatograph Agilent 7890A (Agilent Technologies, US) equipped with a DB-FFAP column and a flame ionization detector. pH was measured with a pH meter (pH meter Basic 20, Crison Instruments, Spain). After liquid sample extraction, withdrawn volumes were replaced with freshly prepared medium.
Cyclic voltammetries (CV) were performed to confirm electrochemical activity. The technique allowed the characterization of electroactive biofilms analyzing changes in the slope of current vs. cathode potential curves, and estimate cathode potentials at which redox reactions are taking place41. CVs were performed using EC-Lab v10.37 software (Bio-Logic Science Instruments, France). Four cycles were done within a range of 0.2 V to − 1.0 V and at a scan rate of 1.0 mV s−1. The obtained CV signals in biotic conditions were compared to abiotic ones. Raw CV data were used for oxidative-reductive peak detection by calculating the first derivative. Analyses were performed using the free-software QSoas42. The mid-point potential (Ef) of redox couples was calculated as the mean value of the oxidative and reductive potential.
Ionic losses were calculated for each medium used. The ionic loss (mV) is related to the electrolyte resistance of the anolyte and catholyte and was estimated according to Ter Heijne et al.43.
During chronoamperometrical operation, power (P, Eq. 1) and energy requirements (E, Eq. 2) were calculated as shown in Eqs. (1) and (2),
being I intensity, and V voltage.
Columbic efficiency (CE) was calculated according to Patil et al.44 (Eq. 3). Ci is the compound i concentration in the liquid phase (mol Ci L−1), ni is the molar conversion factor (2 and 8 eq. mol−1 for H2 and acetate, respectively), F is Faraday’s constant (96,485 C mol e−1), V (L) is the net liquid volume of the cathode compartment, and I is the intensity demand of the system (A).
H2 production rates in all conditions and strains were calculated as a linear response covering the first 25 min of operation according to Tremblay and co-workers19. Linear regressions were calculated for this time-period using SigmaPlot version 11.0 (Systat Software, USA, www.systatsoftware.com) and H2 production rates were obtained from the slope. Unpaired t-tests were used to evaluate statistical significance between biotic and abiotic H2 production rates, current demand, or energy consumption.
DNA extractions and 16S rRNA gene determinations
Samples from both biofilm and bulk liquid were collected under anaerobic conditions during the growth of bacteria and under BES operation. For biofilm measurements, pieces of carbon cloth electrode (1.5 cm2 each) were taken directly using sterile forceps and scissors. For bulk measurements, 10 mL samples were centrifuged (4,400 rpm, 15 min, 4 °C) and supernatants discarded. Both electrode and pelleted cells were stored at − 20 °C until DNA extraction.
DNA extraction was performed using a cetyltrimethylammonium bromide (cTAB) based protocol45. DNA concentrations were measured using Qubit 2.0 Fluoremeter (Thermo Fisher Scientific, USA). Previous to qPCR amplification, samples with 1 ng µL−1 or higher were diluted to avoid inhibition due to excess of DNA. qPCR was used to quantify DNA gene copies targeting 16S rRNA in each sample using 341F and 534R primer pair following the conditions described by López-Gutiérrez and co-workers46. Reactions were performed using the LightCycler 480 SYBR Green I Master Mix (Roche Life Science, Switzerland) and a Lightcycler 96 Real-Time PCR instrument. In all cases, two sample volumes, 1 and 2 µL in a 20 µL total volume were used to ensure no inhibition occurred. A tenfold dilutions series (103–107 copies/mL) of a linearized plasmid containing a 16S rRNA gene sequence was used as standard curve. In all cases qPCR efficiencies were above 90%.
Gene copies per unit mL or cm2 in bulk and biofilm samples were calculated considering dilutions and initial sample volume or surface.
References
Nevin, K. P., Woodard, T. L., Franks, A. E., Summers, Z. M. & Lovley, D. R. Microbial electrosynthesis: Feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. MBio 1, e00103-10 (2010).
Lovley, D. R. & Nevin, K. P. Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr. Opin. Biotechnol. 24, 385–390 (2013).
Choi, O. & Sang, B.-I.B.I. Extracellular electron transfer from cathode to microbes: application for biofuel production. Biotechnol. Biofuels 9, 11 (2016).
Lovley, D. R. Electromicrobiology. Annu. Rev. Microbiol. 66, 391–409 (2012).
Pous, N. et al. Monitoring and engineering reactor microbiomes of denitrifying bioelectrochemical systems. RSC Adv. 5, 68326–68333 (2015).
Perona-Vico, E., Blasco-Gómez, R., Colprim, J. S., Puig, S. & Bañeras, L. [NiFe]-hydrogenases are constitutively expressed in an enriched Methanobacterium sp. population during electromethanogenesis. PLoS ONE 14, e0215029 (2019).
Logan, B. E., Rossi, R., Ragab, A. & Saikaly, P. E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 17, 307–319 (2019).
Kracke, F., Vassilev, I. & Krömer, J. O. Microbial electron transport and energy conservation—the foundation for optimizing bioelectrochemical systems. Front. Microbiol. 6, 1–18 (2015).
Batlle-Vilanova, P. et al. Assessment of biotic and abiotic graphite cathodes for hydrogen production in microbial electrolysis cells. Int. J. Hydrogen Energy 39, 1297–1305 (2014).
Puig, S. et al. Tracking bio-hydrogen-mediated production of commodity chemicals from carbon dioxide and renewable electricity. Bioresour. Technol. 228, 201–209 (2017).
Jourdin, L., Freguia, S., Donose, B. C. & Keller, J. Autotrophic hydrogen-producing biofilm growth sustained by a cathode as the sole electron and energy source. Bioelectrochemistry 102, 56–63 (2015).
Liu, Z., Wang, K., Chen, Y., Tan, T. & Nielsen, J. Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nature Catalysis 3, 274–288 (2020).
Ergal, İ et al. The physiology and biotechnology of dark fermentative biohydrogen production. Biotechnol. Adv. 36, 2165–2186 (2018).
Koku, H., Erolu, I., Gunduz, U., Yucel, M. & Turker, L. Aspect of the metabolism of hydrogen production by Rhodobacter sphaeroides. Int J Hydrog. Energy 27, 1315–1329 (2002).
Geelhoed, J. S. & Stams, A. J. M. Electricity-assisted biological hydrogen production from acetate by Geobacter sulfurreducens. Environ. Sci. Technol. 45, 815–820 (2011).
Aulenta, F., Catapano, L., Snip, L., Villano, M. & Majone, M. Linking bacterial metabolism to graphite cathodes: electrochemical insights into the H2-producing capability of Desulfovibrio sp. Chemsuschem 5, 1080–1085 (2012).
Yu, L., Duan, J., Zhao, W., Huang, Y. & Hou, B. Characteristics of hydrogen evolution and oxidation catalyzed by Desulfovibrio caledoniensis biofilm on pyrolytic graphite electrode. Electrochim. Acta 56, 9041–9047 (2011).
Croese, E., Pereira, M. A., Euverink, G. J., Stams, A. J. & Geelhoed, J. S. Analysis of the microbial community of the biocathode of a hydrogen-producing microbial electrolysis cell. Appl. Microbiol. Biotechnol. 92, 1083–1093 (2011).
Tremblay, P. L., Faraghiparapari, N. & Zhang, T. Accelerated h2 evolution during microbial electrosynthesis with Sporomusa ovata. Catalysts 9, 166 (2019).
Deutzmann, J. S., Sahin, M. & Spormann, A. M. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. MBio 6, 1–8 (2015).
Lienemann, M., Deutzmann, J. S., Milton, R. D., Sahin, M. & Spormann, A. M. Mediator-free enzymatic electrosynthesis of formate by the Methanococcus maripaludis heterodisulfide reductase supercomplex. Bioresour. Technol. 254, 278–283 (2018).
Kundu, A., Sahu, J. N., Redzwan, G. & Hashim, M. A. An overview of cathode material and catalysts suitable for generating hydrogen in microbial electrolysis cell. Int. J. Hydrogen Energy 38, 1745–1757 (2013).
Kracke, F. et al. Robust and biocompatible catalysts for efficient hydrogen-driven microbial electrosynthesis. Commun. Chem. 2, 1–9 (2019).
Call, D. & Logan, B. E. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 42, 3401–3406 (2008).
Selembo, P. A., Merrill, M. D. & Logan, B. E. Hydrogen production with nickel powder cathode catalysts in microbial electrolysis cells. Int. J. Hydrogen Energy 35, 428–437 (2010).
Acinas, S. G., Marcelino, L. A., Klepac-Ceraj, V. & Polz, M. F. Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J. Bacteriol. 186, 2629–2635 (2004).
Clauwaert, P. et al. Minimizing losses in bio-electrochemical systems: The road to applications. Appl. Microbiol. Biotechnol. 79, 901–913 (2008).
Carlozzi, P. & Lambardi, M. Fed-batch operation for bio-H2 production by Rhodopseudomonas palustris (strain 42OL). Renew. Energy 34, 2577–2584 (2009).
Gebicki, J., Modigell, M., Schumacher, M., Van Der Burg, J. & Roebroeck, E. Comparison of two reactor concepts for anoxygenic H2 production by Rhodobacter capsulatus. J. Clean. Prod. 18, S36–S42 (2010).
Vilar-Sanz, A. et al. Denitrifying nirK-containing alphaproteobacteria exhibit different electrode driven nitrite reduction capacities. Bioelectrochemistry 121, 74–83 (2018).
Guzman, M. S. et al. Phototrophic extracellular electron uptake is linked to carbon dioxide fixation in the bacterium Rhodopseudomonas palustris. Nat. Commun. 10, 1–13 (2019).
Kim, M. S., Kim, D. H., Cha, J. & Lee, J. K. Effect of carbon and nitrogen sources on photo-fermentative H2 production associated with nitrogenase, uptake hydrogenase activity, and PHB accumulation in Rhodobacter sphaeroides KD131. Bioresour. Technol. 116, 179–183 (2012).
Wu, S. C., Liou, S. Z. & Lee, C. M. Correlation between bio-hydrogen production and polyhydroxybutyrate (PHB) synthesis by Rhodopseudomonas palustris WP3-5. Bioresour. Technol. 113, 44–50 (2012).
Bose, A., Gardel, E. J., Vidoudez, C., Parra, E. A. & Girguis, P. R. Electron uptake by iron-oxidizing phototrophic bacteria. Nat. Commun. 5, 3391 (2014).
Aryal, N., Tremblay, P. L., Lizak, D. M. & Zhang, T. Performance of different Sporomusa species for the microbial electrosynthesis of acetate from carbon dioxide. Bioresour. Technol. 233, 184–190 (2017).
Nevin, K. P. et al. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 77, 2882–2886 (2011).
Lojou, E., Durand, M. C., Dolla, A. & Bianco, P. Hydrogenase activity control at Desulfovibrio vulgaris cell-coated carbon electrodes: biochemical and chemical factors influencing the mediated bioelectrocatalysis. Electroanalysis 14, 913–922 (2002).
Guiral-Brugna, M., Giudici-Orticoni, M.-T., Bruschi, M. & Bianco, P. Electrocatalysis of the hydrogen production by [Fe] hydrogenase from Desulfovibrio vulgaris Hildenborough. J. Electroanal. Chem. 510, 136–143 (2001).
Cordas, C. M., Moura, I. & Moura, J. J. G. Direct electrochemical study of the multiple redox centers of hydrogenase from Desulfovibrio gigas. Bioelectrochemistry 74, 83–89 (2008).
Deutzmann, J. S. & Spormann, A. M. Enhanced microbial electrosynthesis by using defined co-cultures. ISME J. 11, 704–714 (2017).
Harnisch, F. & Freguia, S. A basic tutorial on cyclic voltammetry for the investigation of electroactive microbial biofilms. Chem. Asian J. 7, 466–475 (2012).
Fourmond, V. QSoas: a versatile software for data analysis. Anal. Chem. 88, 5050–5052 (2016).
Ter Heijne, A., Hamelers, H. V. M., De Wilde, V., Rozendal, R. A. & Buisman, C. J. N. A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ. Sci. Technol. 40, 5200–5205 (2006).
Patil, S. A. et al. A logical data representation framework for electricity-driven bioproduction processes. Biotechnol. Adv. 33, 736–744 (2015).
Llirós, M., Casamayor, E. O. & Borrego, C. High archaeal richness in the water column of a freshwater sulfurous karstic lake along an interannual study. FEMS Microbiol. Ecol. 66, 331–342 (2008).
López-Gutiérrez, J. C. et al. Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J. Microbiol. Methods 57, 399–407 (2004).
Acknowledgments
This study has received funding from the European Union’s Horizon 2020 research and innovation program under the grant agreement no 760431. LEQUIA and IEA have been recognized as a consolidated research groups by the Catalan Government (2017-SGR-1552 and 2017SGR-548, respectively). E. P.-V. is grateful for the Research Training grant from the University of Girona (IFUdG2018/52). S.P. is a Serra Hunter Fellow (UdG-AG-575) and acknowledges the funding from the ICREA Academia award.
Author information
Authors and Affiliations
Contributions
Experiments were designed by L.B. and S.P. and L.F.-P. and E.P.-V. performed the experiments. Data analysis was by L.F.-P. and E.P.-V. with help from L.B. and S.P. Manuscript was reviewed and edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perona-Vico, E., Feliu-Paradeda, L., Puig, S. et al. Bacteria coated cathodes as an in-situ hydrogen evolving platform for microbial electrosynthesis. Sci Rep 10, 19852 (2020). https://doi.org/10.1038/s41598-020-76694-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76694-y
This article is cited by
-
Rewiring photosynthetic electron transport chains for solar energy conversion
Nature Reviews Bioengineering (2023)
-
Metatranscriptomic insights into the microbial electrosynthesis of acetate by Fe2+/Ni2+ addition
World Journal of Microbiology and Biotechnology (2023)
-
Co anchored on porphyrinic triazine-based frameworks with excellent biocompatibility for conversion of CO2 in H2-mediated microbial electrosynthesis
Frontiers of Chemical Science and Engineering (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.