Abstract
Chrono-biological traits were changed by selecting for life-history traits via a genetic linkage controlling both time-related behavioural and life-history traits. Behavioural traits were compared between lines selected for young (Y-lines) and old (O-lines) age at reproduction in the melon fly, Bactrocera cucurbitae (Coquillett). Adults from O-lines, which survive longer than flies from Y-lines, mated later in the day and had a longer period of circadian rhythm in the locomotor activity than those from Y-lines. Flies from F1 reciprocal crosses had an intermediate time of mating and periods of circadian rhythm between that of the parents, indicating a genetic basis to these traits. The presence of these behavioural differences across the selection lines indicates that chrono-biological traits exhibit correlated responses to selection on age at reproduction. The correlated responses in the behavioural traits to selection for life-history traits are discussed from two points of view: pleiotropy and inadvertent selection.
Similar content being viewed by others
Introduction
Many artificial selections for age at reproduction have been done to clarify the evolutionary mechanism of ageing (see reviews in Finch, 1990; Curtsinger et al, 1995; Zwaan, 1999). Animals selected for age at reproduction include Drosophila melanogaster (Luckinbill et al, 1984; Rose, 1984; Partridge and Fowler, 1992; Chippindale et al, 1994; Partridge et al, 1999), D. subobscura (Wattiaux, 1968), Tribolium castaneum (Mertz, 1975), Acanthoscedelis obtectus (Tucic et al, 1996), and Bactrocera cucurbitae (Miyatake, 1997a). Analyses of correlated responses to selection are useful because they can contribute to an understanding of physiological mechanisms involved in the response to selection (Gibbs, 1999; Harshman and Hoffmann, 2000). Most studies on correlated responses to selection for age at reproduction have concentrated on life-history traits such as starvation and desiccation resistances (eg, Rose, 1984; Roper et al, 1993; Chippindale et al, 1994; Partridge et al, 1999) and physiological traits (eg, Luckinbill et al, 1984; Rose, 1984; Service et al, 1985; Force et al, 1995). In contrast, only three studies, all on mating frequencies, have focused on behavioural traits as the correlated response (Service, 1993; Pletcher et al, 1997; Sgró et al, 2000).
Recently, mutants pleiotropically affecting the period lengths of biological clock, life-history, and behavioural events have been found in D. melanogaster (Kyriacou and Hall, 1980; Kyriacou et al, 1990), Paramecium bursaria (Tokushima et al, 1994; Miwa and Yajima, 1995) and Caenorhabditis elegans (Wong et al, 1995; Lakowski and Hekimi, 1996). If a gene controlling biological rhythm is influenced by selection for life-history traits, some chrono-biological traits such as circadian rhythm and time of mating may also be changed via pleiotroic gene action. Age at reproduction is unquestionably a time-related trait in a life. Therefore, there will be a high possibility of changing chrono-biological traits as correlated responses to selection for age at reproduction. If chrono-biological traits have changed as the correlated response to selection for age at reproduction, the speed of ageing should be affected by the correlation. However, no study has focused on genetic correlations between the chrono-biological traits and age at reproduction.
In this paper, it is shown that a selection for age at reproduction alters positively the time of mating in a day and the period of circadian rhythm in the melon fly, B. cucurbitae (Coquillett). Lines selected for younger age at reproduction have earlier time of mating and shorter circadian period, whereas lines selected for older age at reproduction have a later time of mating and longer circadian period. B. cucurbitae is a good animal to use for examining the time of mating and circadian rhythm because the methods for analysing these traits have been established (Miyatake, 1997b; Shimizu et al, 1997), and three replicated lines selected for age at reproduction have been cultivated for over 15 generations (Miyatake, 1997a).
Materials and methods
Selection lines
The base population for the selection was a mass-reared strain that has been maintained for 63 generations in the Okinawa Prefectural Fruit Fly Eradication Project Office, Okinawa, Japan, according to the method described by Nakamori et al (1992). This strain originated in 1985 with 19281 larvae collected from bitter gourd fruits, Momordica charantia var. pavel Crantz, collected from the southern part of Okinawa Island Japan (Kakinohana, 1996).
Three replicate ‘young’ and ‘old’ lines were produced from the base population. Selection was started in June 1992 and was initiated from about 3000 pupae of the mass-reared strain. These pupae were divided into three populations, and each was placed in a rearing cage (40 by 30 by 28 cm). The adults (ca. 1000 flies) were allowed to eclose in each cage. When these adults were 10 to 15 days and 55 to 60 days old, eggs were collected from flies of each cage for 18 h (from 16.00 to 10.00 the next morning) by using artificial oviposition cylinders. Lines originating from the eggs collected at different ages were named young lines (Y-lines) and old lines (O-lines). About 2400 eggs (0.3 ml) were placed on 300 g of larval medium (Nakamori et al, 1992) in a sample container (130 mm diameter, 92 mm height) for each line. Each cup was kept separately in a larger sample container (150 mm diameter, 92 mm height) filled with water (80 ml). Water was exchanged for a pupariation substrate consisting of a 7:1 mixture of sawdust and water 4 days after egg seeding. Mature larvae popped out from the medium and pupated in the pupation substrate. The pupae were put into a plastic cup (200 ml) and set in each rearing cage. These selection regimes were continued for 65 generations for Y-lines and 24 generations for O-lines. Selection and all the experiments were conducted in a laboratory at 25°C under a photoperiod of 14:10 (L:D) h (light phase 05.30 to 19.30). For further details of the selection procedure and the consequent changes in longevity, oviposition curves, developmental periods, and survival rates, see Miyatake (1997a).
Time of mating
Time of mating (the time when copulation begins) was examined in the 42nd generation of Y-lines and the 15th generation of O-lines under light:dim laboratory lighting. The light intensity was 350 lx (2 lx) in light (dim) conditions. Adults emerging on the same day were sexed within 3 days after emergence. Males and females were kept separately in cages (20 × 20 × 30 cm) in a laboratory at 26 ± 1°C under a photoperiod of 14:10 (L:D) h. About 25 days after adult emergence, time of mating was examined in the laboratory. A male and a female were released into a transparent plastic cup (80 mm diameter, 40 mm height) in which food and water were provided. Fifty pairs were set up for Y-1, Y-2, Y-3, O-1, O-2, and O-3 lines. Flies were released into the cup from 10 to 9 h before lights-down to accustom them to the cup, and mating pairs were counted for 11 h at 15-min intervals from 3 h before lights-down.
At generation 66 for the Y-lines and 25 for the O-lines, reciprocal crosses were made between the Y and O lines. Times of mating in the F1 flies (Y females × O males, O females × Y males, Y females × Y males, and O females × O males) were measured using the same methods as described above.
The sequential Bonferroni method (Rice, 1989) was used to compare the number of mated pairs among populations. The Scheffe's methods were used to compare mating times among populations (SAS Institute, 1998).
Circadian rhythm
Monitoring methods are described in Shimizu et al (1997). Briefly, adults were kept singly in containers (36 mm diameter, 66 mm height) and provided with water and sugar. Their locomotor activities were monitored by interruptions of an infrared beam and a photoelectric switch (OMRON, Tokyo). Signals of interruptions were sent to a computer (NEC, Tokyo) and the numbers recorded in 6-min intervals. The free-running period was computed by the least-square spectrum (Chiba and Takahashi, 1991) from free-running data for 2 weeks in DD (continuous darkness) after a week of entrainment to 14 h L: 10 h D. The adult age at the transfer from 14 h L: 10 h D to D:D varied from 2 to 3 weeks. A total of 135 out of 182 flies examined (86 Y-line flies and 96 O-line flies) exhibited free-running rhythms. The remaining 47 flies died or did not demonstrate free-running rhythms clearly enough for calculating the periods. All analyses were performed using SAS PROC GLM (SAS Institute, 1988), calculating type III with Strain and Replication as fixed effects and random error nested within Replication.
Results
Time of mating
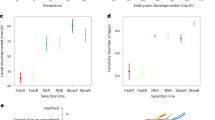
Flies from the three replicated Y-lines mated significantly earlier than those from the O-lines (Figure 1, Table 1). Crosses between lines also showed that offspring from Y-parents (Y × Y flies) mated significantly earlier than those from O-parents (O × O flies) (Figure 2, Table 2). The reciprocal cross flies had intermediate time of mating between Y × Y and O × O flies (Figure 2, Table 2).
Time course of the cumulative fraction of mated pairs in crosses between young lines and between old lines within each selection regime (upper figure), and in the reciprocal cross lines between young and old lines (bottom figure) of Bactrocera cucurbitae. In the upper figure, solid (dashed) lines show young (old) replicated lines. In the bottom figure, solid (dashed) lines show offspring from O females × Y males (Y females × O males). Hollowed (dotted) horizontal bars represent light (dim) phases.
A significantly lower frequency of mated pairs was observed in O-line flies than in Y-line flies when mating was allowed within a selected line (Table 1). However, no difference was found in the percentages of mated pairs per day among flies from Y- and O-lines when the crosses between selected lines were used (Table 2). This suggests that depression by inbreeding occurred in mating activity only in old-line flies. The selection regime for old lines may suffer inbreeding depression, because the number of surviving flies in age at reproduction is smaller in old lines than in young lines.
Circadian rhythm
The mean free-running periods in the lines tested are shown in Table 3. There was an association between the free-running period and selected regime in males and females (Table 4). The period of free-running rhythm was shortened by selection for young age at reproduction, and lengthened by selection for old age at reproduction. Free-running periods also varied among replicated lines, and thus there was a significant interaction between the selected regime and selection replication only in females (Table 4).
Discussion
Adults from Y-lines had earlier mating time in the day and had a shorter period of circadian rhythm in the locomotor activity than those from O-lines. The presence of these differences across the selection lines indicates that chrono-biological traits show correlated responses to selection on age at reproduction.
There may be two possible explanations for the correlated responses. The first is the genetic correlation via a gene pleiotropically controlling age at reproduction, longevity, and circadian period. In this case, there is a genetic correlation between age at reproduction and circadian period. Generally, when the circadian clock ticks faster, events controlled by the circadian clock (eg, time of mating and adult eclosion) occur earlier as well (Pittendrigh, 1981). Many life events, such as age at reproduction and longevity, might be controlled by common physiological speed such as passing speed of some materials through cell membranes. In Drosophila, the gene (per) slowing down the ticking of the circadian clock also slows down the periodic fluctuation of the courtship song (Kyriacou and Hall, 1980) and larval development (Kyriacou et al, 1990). In Paramecium bursaria, strains and a mutant that mature at a small number of fissions exhibit fast ticking of the circadian clock, swimming, and contractile vacuole contraction (Tokushima et al, 1994; Miwa and Yajima, 1995). In Caenorhabditis elegans, a gene that slows down embryonic cell cycles, also slows development, ageing, and several adult behaviours, for example, swimming, pharyngeal pumping, and defecation (Wong et al, 1995; Lakowski and Hekimi, 1996). In B. cucurbitae, therefore, the parallel change observed between the circadian rhythm and the time of mating, as a correlated response to the selection for age at reproduction, may be explained as a pleiotropic consequence via an underlying gene.
The second explanation for the correlated change in circadian period is that it is due to inadvertent selection on development time (Harshman and Hoffmann, 2000). In this case, there may be no genetic correlation between age at reproduction and the circadian period. In B. cucurbitae, a gene pleiotropically controlling developmental and circadian periods has been found (Shimizu et al, 1997), that is, shorter developmental period lines have shorter circadian periods. The selections for younger age at reproduction cause the decreased development time, probably because of an inadvertent selection (Miyatake, 1997a). Namely the flies, which matured sexually earlier with short larval periods, were favoured by the selection regime for the young lines. Thus, there may be a possibility that the shortened development time in lines selected for young age at reproduction causes shorter circadian periods and earlier mating times than lines selected for old age at reproduction with a long developmental period. In Drosophila, selection for young age at reproduction sometimes causes the decreased pre-adult period (Roper et al, 1993; Chippindale et al, 1994). This decreased pre-adult period in lines selected for young age at reproduction was caused by an inadvertent selection on selected lines resulting in an apparent correlated response (Partridge et al, 1999).
In both the above explanations, genes controlling clock traits are selected through selections for age at reproduction. In D. melanogaster, many artificial selections for age at reproduction have been done to test ageing (Zwaan, 1999). Ageing may be a trait controlled by ‘time’. In B. cucurbitae, flies from Y-lines have shorter longevity as a result of correlated response to selection for younger age at reproduction, whereas flies from O-lines have longer longevity due to selection for older age at reproduction (Miyatake, 1997a). It will be appropriate to examine the change in chronobiological traits expressed from clock genes in strains selected for age at reproduction, and to focus on the relationship between ageing and clock genes in D. melanogaster.
References
Chiba, Y, Takahashi, K (1991). Chronobiology Handbook. Asakura shoten: Tokyo (in Japanese).
Chippindale, AK, Hoang, DT, Service, PM, Rose, MR (1994). The evolution of development in Drosophila melanogaster selected for postponed senescence. Evolution, 48: 1880–1899.
Curtsinger, JW, Fukui, HH, Khazaeli, AA, Kirscher, A, Pletcher, SD, Promislow, DEL, Tatar, M (1995). Genetic variation and aging. Ann Rev Genet, 29: 553–575.
Finch, CE (1990). Longevity, Senescence, and the Genome. The University of Chicago Press: Chicago.
Force, AG, Staples, T, Soliman, S, Arking, R (1995). Comparative biochemical and stress analysis of genetically selected Drosophila strains with different longevities. Dev Gen, 17: 340–351.
Gibbs, AG (1999). Laboratory selection for the comparative physiologist. J Exp Biol, 202: 2709–2718.
Harshman, LG, Hoffmann, AA (2000). Laboratory selection experiments using Drosophila: what do they really tell us? Trend Ecol Evol, 15: 32–36.
Kakinohana, H (1996). Studies on the mass production of the melon flies, Bactrocera cucurbitae Coquillett. Bull Okinawa Agric Expt Stn, 16: 1–102. (in Japanese with English summary).
Kyriacou, CP, Hall, JC (1980). Circadian rhythm mutations in Drosophila affect short-term fluctuations in the male's courtship song. Proc Natl Acad Sci USA, 77: 6929–6933.
Kyriacou, CP, Oldroyd, M, Wood, J, Sharp, M, Hill, M (1990). Clock mutations alter developmental timing in Drosophila. Heredity, 64: 395–401.
Lakowski, B, Hekimi, S (1996). Determination of life-span in Caenorhabditis elegans by four clock genes. Science, 272: 1010–1013.
Luckinbill, LS, Arking, R, Clare, MJ, Cirocco, WC, Buck, SA (1984). Selection for delayed senescence in Drosophila melanogaster. Evolution, 38: 996–1003.
Mertz, DB (1975). Senescent decline in flour beetle strains selected for early adult fitness. Physiol Zool, 48: 1–23.
Miyatake, T (1997a). Genetic trade-off between early fecundity and longevity in Bactrocera cucurbitae (Diptera: Tephritidae). Heredity, 78: 93–100.
Miyatake, T (1997b). Correlated responses to selection for developmental period in Bactrocera cucurbitae (Diptera: Tephritidae): time of mating and daily activity rhythms. Behav Genet, 27: 489–498.
Miwa, I, Yajima, H (1995). Correlation of the period length of circadian rhythms with the length of immaturity in Paramecium bursaria. Zool Sci, 12: 53–59.
Nakamori, H, Kakinohana, H, Yamagishi, M (1992). Automated mass production system for fruit flies based on the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae). In: Anderson TE, Leppla NC (eds) Advances in Insect Rearing for Research and Pest Management, Westview Press: Boulder, CO. pp 441–454.
Partridge, L, Fowler, K (1992). Direct and correlated responses to selection on age at reproduction in Drosophila melanogaster. Evolution, 46: 76–91.
Partridge, L, Prowse, N, Pignatelli, P (1999). Another set of responses and correlated responses to selection on age at reproduction in Drosophila melanogaster. Proc R Soc Lond B, 266: 255–261.
Pittendrigh, CS (1981). Circadian systems: Entrainment. In: Aschoff J (ed) Handbook of Behavioral Neurobiology, vol 4, Biological Rhythms Plenum Press: New York. pp 95–124.
Pletcher, SD, Fukui, HH, Curtsinger, JW (1997). Mating behavior in Drosophila melanogaster selected for altered longevity. Evolution, 51: 303–307.
Rice, WR (1989). Analyzing tables of statistical tests. Evolution, 43: 223–225.
Roper, C, Pignatelli, P, Partridge, L (1993). Evolutionary effects of selection on age at reproduction in larval and adult Drosophila melanogaster. Evolution, 47: 445–455.
Rose, MR (1984). Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution, 38: 1004–1010.
SAS Institute (1988). SAS/STAT user's guide, release 6.03 edition, SAS Institute, Cary, NC.
SAS Institute (1998). Statview J.5.0. SAS Institute, Cary, NC.
Service, PM (1993). Laboratory evolution of longevity and reproductive components in male fruit flies: mating ability. Evolution, 47: 387–399.
Service, PM, Hutchinson, EW, Mackinley, MD, Rose, MR (1985). Resistance to environmental stress in Drosophila melanogaster selected for postponed senescence. Rhysiol Zool, 58: 380–389.
Sgró, CM, Geddes, G, Fowler, K, Partridge, L (2000). Selection on age at reproduction in Drosophila melanogaster: female mating frequency as a correlated response. Evolution, 54: 2152–2155.
Shimizu, T, Miyatake, T, Watari, Y, Arai, T (1997). A gene pleiotropically controlling developmental and circadian periods in the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae). Heredity, 70: 600–605.
Tokushima, H, Okamoto, KI, Miwa, I, Nakaoka, Y (1994). Correlation between circadian periods and cellular activities in Paramecium bursaria. J Comp Physiol A, 175: 767–772.
Tucic, N, Gliksman, L, Seslija, D, Milanovuc, D, Mikuljanac, S, Stojokovic, O (1996). Laboratory evolution of longevity in the bean weevil (Acanthoscelides obtectus). J Evol Biol, 9: 485–503.
Wattiaux, JM (1968). Cumulative parental age effects in Drosophila subobscura. Evolution, 22: 406–421.
Wong, A, Boutis, P, Hekimi, S (1995). Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioural timing. Genetics, 139: 1247–1259.
Zwaan, BJ (1999). The evolutionary genetics of ageing and longevity. Heredity, 82: 589–597.
Acknowledgements
I thank Dr Kenjiro Kawasaki (National Institute of Sericultural and Entomological Science, Japan) for useful comments on the manuscript, Dr Toru Shimizu (Ryukyusankei Co Ltd, Japan) for technical advice on the calculation of free-running periods, and Dr Takuro Oikawa (Faculty of Agriculture, Okayama University, Japan) for statistical advice. This study was conducted at the Okinawa Prefectural Fruit Fly Eradication Office, Okinawa, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyatake, T. Circadian rhythm and time of mating in Bactrocera cucurbitae(Diptera: Tephritidae) selected for age at reproduction. Heredity 88, 302–306 (2002). https://doi.org/10.1038/sj.hdy.6800044
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800044
Keywords
This article is cited by
-
Correlated changes in circadian clocks in response to selection for faster pre-adult development in fruit flies Drosophila melanogaster
Journal of Comparative Physiology B (2013)
-
Rhythmic male reproductive behavior controls timing of courtship and mating in Laupala cerasina
Behavioral Ecology and Sociobiology (2012)
-
Comparison of two polymorphic sites in the clock gene cryptochrome in the Taiwan strain of the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae): a possible quick method to estimate the mating time of trapped invading flies
Applied Entomology and Zoology (2011)
-
Insect quality control: synchronized sex, mating system, and biological rhythm
Applied Entomology and Zoology (2011)
-
Genetic basis of incidence and period length of circadian rhythm for locomotor activity in populations of a seed beetle
Heredity (2010)