Abstract
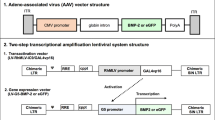
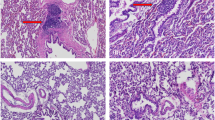
Previous reports have suggested that bone morphogenetic protein (BMP) gene therapy could be applied for in vivo bone regeneration. However, these studies were conducted either using immunodeficient animals because of immunogenicity of adenovirus vectors, or using ex vivo gene transfer technique, which is much more difficult to handle. Adeno-associated virus (AAV) is a replication-defective virus without any association with immunogenicity and human disease. This study was conducted to investigate whether orthotopic new bone formation could be induced by in vivo gene therapy using AAV-based BMP2 vectors. To test the feasibility of this approach, we constructed an AAV vector carrying human BMP2 gene. Mouse myoblast cells (C2C12) transduced with this vector could produce and secrete biologically active BMP2 protein and induce osteogenic activity, which was confirmed by ELISA and alkaline phosphatase activity assay. For in vivo study, AAV-BMP2 vectors were directly injected into the hindlimb muscle of immunocompetent Sprague–Dawley rats. Significant new bone under X-ray films could be detected as early as 3 weeks postinjection. The ossification tissue was further examined by histological and immunohistochemical analysis. This study is, to our knowledge, the first to establish the feasibility of AAV-based BMP2 gene therapy for endochondral ossification in immunocompetent animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Wozney JM, Rosen V . Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop 1998; 346: 26–37.
Chen Y . Orthopaedic applications of gene therapy. J Orthop Sci 2001; 6: 199–207.
Sandhu JS et al. Effect of interleukin-6 secreted by engineered human stromal cells on osteoclasts in human bone. Bone 1999; 24: 217–227.
Breitbart AS et al. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg 1999; 42: 488–495.
Engstrand T et al. Transient production of bone morphogenetic protein 2 by allogeneic transplanted transduced cells induces bone formation. Hum Gene Ther 2000; 11: 205–211.
Alden TD et al. In vivo endochondral bone formation using a bone morphogenetic protein-2 adenoviral vector. Hum Gene Ther 1999; 10: 2245–2253.
Franceschi RT, Wang D, Krebsbach PH, Rutherford RB . Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Chem 2000; 78: 476–486.
Lieberman JR et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg 1999; 81A: 905–917.
Chen Y et al. In vivo new bone formation by direct transfer of adenoviral mediated bone morphogenetic protein-4 gene. Biochem Biophys Res Commun 2002; 298: 121–127.
Jiang ZL et al. Local high-capacity adenovirus-mediated mCTLA4Ig and mCD40Ig expression prolongs recombinant gene expression in skeletal muscle. Mol Ther 2001; 3: 892–900.
Monahan PE, Samulski RJ . Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today 2000; 6: 433–440.
Rabinowitz JE, Samulski J . Adeno-associated virus expression systems for gene transfer. Curr Opin Biotechnol 1998; 9: 470–475.
Muzyczka N . Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol 1992; 158: 97–129.
Samulski RJ, Chang LS, Shenk T . Helper-free stocks of recombinant adeno-associated viruses: nor malintegration does not require viral gene expression. J Virol 1989; 63: 3822–3828.
Xiao X, Li J, Samulski RJ . Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol 1996; 70: 8098–8108.
Katagiri T et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 1994; 127: 1755–1766.
Wilson JM . Adenovirus as gene-delivery vehicles. N Engl J Med 1996; 334: 1185–1187.
Apparailly F et al. Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum Gene Ther 2002; 13: 1179–1188.
Grimm D, Kern A, Rittner K, Kleinschmidt JA . Novel tools for production and purification of recombinant adeno-associated virus vectors. Hum Gene Ther 1998; 9: 2745–2760.
Auricchio A et al. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther 2001; 12: 71–76.
Acknowledgements
We thank the Laboratory Animal Unit, The University of Hong Kong for helping us with animal holding and maintenance. Also, we are grateful to Mr David Wilmshurst, the technical writer in The University of Hong Kong, for his kind review on this manuscript. This study was supported by a University Research Grant (2000–2001) from The University of Hong Kong.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, Y., Luk, K., Cheung, K. et al. Gene therapy for new bone formation using adeno-associated viral bone morphogenetic protein-2 vectors. Gene Ther 10, 1345–1353 (2003). https://doi.org/10.1038/sj.gt.3301999
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301999
Keywords
This article is cited by
-
Ubiquitin-specific protease 53 promotes osteogenic differentiation of human bone marrow-derived mesenchymal stem cells
Cell Death & Disease (2021)
-
Limited potential of AAV-mediated gene therapy in transducing human mesenchymal stem cells for bone repair applications
Gene Therapy (2021)
-
Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold
Stem Cell Research & Therapy (2019)
-
BMP2 gene delivery to bone mesenchymal stem cell by chitosan-g-PEI nonviral vector
Nanoscale Research Letters (2015)
-
Two-stage therapeutic utility of ectopically formed bone tissue in skeletal muscle induced by adeno-associated virus containing bone morphogenetic protein-4 gene
Journal of Orthopaedic Surgery and Research (2015)