Q1. What is wrong with the oral preparatory phase?
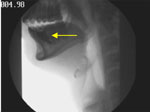
Figure 1 is a snapshot of the oral phase of swallowing from the patient's VFSS. Note that the bolus is in the anterior floor-of-mouth and not on the dorsum of the tongue. Although the bolus in this position may be normal in an elderly subject, it is quite a rare occurrence in young adults. The dynamic imaging (Video 1) shows the patient is unable to form a bolus and position it on the dorsum of the tongue owing to weakness of the tongue.
Q2. What muscles and cranial nerves are involved in oral preparatory phase of swallowing?
The muscles of mastication are innervated by the trigeminal cranial nerve V, facial muscles are innervated by the facial cranial nerve VII, and the tongue is innervated by the hypoglossal cranial nerve (XII). This patient has paresis of cranial nerves V, VII, and XII. It is not shown in this study, but he also has drooling. This produces poor bolus containment and control with impaired propulsion of the bolus posteriorly to the region of the faucial pillars, where activation of the swallowing response may be initiated.
Q3. Does the patient have premature spill of barium into the pharynx?
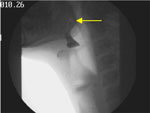
Yes, essentially. As shown in Figure 2 (a snapshot from the patient's VFSS), premature spill occurs owing to disorganized or absent tongue actions with incompetence of the glossopalatal seal and is characterized by movement of barium posteriorly, without cohesive organized intent, prior to the onset of a swallow reflex. Premature spill is attributed to weakness of the posterior tongue or the soft palate or both. The nerves involved are XII, X and spinal accessory XI. The small amount of barium (3 mL) assisted the patient in not revealing more dramatic premature spillage.
Q4. Is a swallow response present in this patient?
This study (Video 1) shows what now appears to be feeble but organized activity of the "leading complex" (e.g., mylohyoid, anterior belly digastric, levator veli palatine) muscles during swallowing, indicating that the neural arc of the reflexive swallow is not completely destroyed and the muscles are not totally paralyzed. It is important to realize that nonpatterned movements of the hyoid, larynx, soft palate, and tongue may occur during attempted swallows when the sensory limb of the neural arc of the swallowing reflex is destroyed.
The VFSS identifies the onset of the swallow response by the time point when the head of the bolus appears at the ramus of the mandible as it crosses the tongue. However, with premature spillage, barium reaches the pharynx well before initiation of a swallow.
Q5. Does the patient have normal velopharyngeal closure or nasal regurgitation?
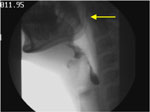
As shown in Figure 3 (a snapshot from the patient's VFSS), the velopharyngeal closure is impaired. At this time actual nasal regurgitation of barium is not observed.
Q6. What nerves and muscles are involved in velopharyngeal closure?
Velopharyngeal closure is accomplished by contraction of the superior pharyngeal constrictors and elevation of the soft palate (levator veli palatini), which are supplied by the cranial nerve X.
Q7. Does this study show tracheal aspiration?
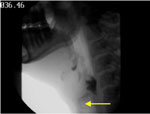
Figure 4 (a snapshot from the patient's VFSS) shows that barium entered the unprotected larynx and the trachea during a swallow. The patient aspirated a trace amount at the end of the sequence in the lateral view and again later in the anterior-posterior view. The focus is on the inferior margin of the cricoid anteriorly (yellow arrow) as a more reliable marker for aspiration on VFSS. The patient responded to the aspiration with a productive cough, and the later aspiration resulted in cough behavior.
Q8. What mechanisms normally prevent aspiration?
Normally, aspiration is prevented by a number of mechanisms including (1) prevention of premature spill; (2) prompt and organized transfer of food through the oral cavity and pharynx owing to timely and forceful contraction of the posterior tongue and the sequential contraction of the pharyngeal muscles and prompt opening of the upper sphincter as part of the sequential swallow; (3) careful coordination of swallowing with respiration and speech; and (4) protective reflexes against aspiration, including (a) laryngeal elevation, lifting up and forward movement of the larynx so as to move out of the way and "hide" under the epiglottis and the tongue during a swallow, and (b) reflex closure of the larynx true and false vocal cords along with the aryepiglottic folds (bottom to top); epiglottis inversion helps direct bolus around the airway during swallowing. If these fail and food material enters the trachea, a strong cough reflex is normally triggered. Airway clearance can also be achieved later by ciliary action or the alveolar macrophage.
Q9. Enumerate the mechanistic abnormalities that may be predisposing to aspiration in this patient.
- Premature spill
- Ineffective vs. absent swallow reflex response
- Pharyngeal stasis owing to pharyngeal paralysis
- Pharyngeal stasis owing to impaired opening of the UES
- Poor posterior tongue contraction
- Poor hyolaryngeal elevation
- Paralysis of the vocal cords
Q10. What is the evidence of pharyngeal paresis?
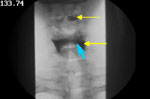
In Figure 5 there is clear evidence of bilateral pharyngeal weakness and paralysis as evidenced by stasis of barium in the vallecula and the pyriform sinuses (yellow arrows) and the lack of movement of the pharyngeal wall.
Figure 5: Evidence for barium stasis in valleculae and pyriforms (yellow thin arrows).
Also evidence of the posterior surface of the cricoid cartilage opposed to the posterior pharyngeal wall that constitutes the upper sphincter (blue short arrow).
Q11. What muscles and nerves are responsible for pharyngeal propulsion?
The pharyngeal wall consists of overlapping superior middle and inferior constrictors on its posterior aspects and sides. Anteriorly, there is the larynx, epiglottis, and posterior part of the tongue. The pharyngeal muscles and the posterior tongue assisted from the palatoglossus (X, XI) and are responsible for pharyngeal propulsion of the bolus. The pharyngeal constrictors are innervated by the branches of the vagus (X), whose motor nuclei arise in the nucleus ambiguus.
Q12. Does the UES open in this study?
In Figure 5 the upper esophageal sphincter does not open at any time and no barium is seen to enter the esophagus. The enlarged pyriform sinuses can mask the impaired opening of the sphincter in the lateral view; however, it is well seen in the anteroposterior view (Figure 5) that the posterior surface of the cricoid cartilage remains opposed to the posterior pharyngeal wall that constitutes the upper sphincter (blue short arrow).
Q13. What are the unique features of the upper esophageal sphincter (UES)? How does this differ from the closure of the lower esophageal sphincter (LES)?
Classically defined sphincters such as in the pupil are valve-like structures that can close and open. Muscles that close the sphincters are called constrictor muscles and those that open or dilate are called the dilator muscles. The UES has muscles only in its posterior wall and sides, and its anterior wall is cartilaginous. Thus its designation as a sphincter is a topic of discussion. The maintained closure of the upper sphincter is owing to two separate mechanisms: (1) mechanical pressure of the cartilaginous anterior wall, and (2) tonic contraction of its striated muscles owing to tonic excitation of its motor nerve. The mechanical component of the upper sphincter keeps it closed and provides resistance to flow even when the sphincter muscle is paralyzed. This is in contrast to the LES, which is composed of circular smooth muscles that have an inherent property of staying contracted. They have no mechanical component to the closure, and when the lower sphincter muscle is completely paralyzed, it generates no intraluminal pressure and provides no resistance to flow across it.
Q14. What is the difference between opening and relaxation? What is the mechanism of UES opening? How do opening and relaxing of the upper esophageal sphincter (UES) differ from that in the lower esophageal sphincter (LES)?
Opening is the physical separation of the walls (of the sphincter), whereas relaxation is loss of tension or loosening. Relaxation alone may not cause opening, but relaxation of the sphincter muscles is an important prerequisite for opening. The UES relaxation is owing to loss of tension in the cricopharyngeus muscle, and its opening requires flow across its lumen by the pharyngeal contraction. However, the UES opening may also occur by the anterior movement of the cricoid cartilage via contraction of the suprahyoid and infrahyoid muscles. These muscle groups may be considered as the dilators of the UES. Normally, during swallowing the cricopharyngeus (CP) relaxes and suprahyoid muscles contract, leading to full opening of the UES. When suprahyoid muscles contract normally during a swallow, but the cricopharyngeus fails to relax, the UES is seen in the open state, with its lumen compromised by the nonrelaxing CP muscle that sticks out as a CP bar. The lack of relaxation of the CP muscle may be owing to overriding reflex contraction and the loss of central inhibition of motor neurons to the CP muscle or fibrosis. Because the CP is a striated muscle, unlike the smooth muscle of the LES, it does not receive direct inhibitory innervation.
In contrast, the LES has no dilator fibers the can open it. The opening of the LES is possible only with relaxation of the LES muscle. Unlike the UES, which is striated muscle, the LES is composed of smooth muscle fibers. Relaxation of the LES is the result of direct inhibitory nerves that act by releasing nitric oxide as the neurotransmitter. The opening of the LES is the result of movement of the bolus across the relaxed sphincter
Q15. What muscles and nerves are responsible for UES opening?
The upper sphincter opening is the result of anterior movement of the cricoid cartilage and the larynx in concert with inhibitory input to the CP from the swallow center via the pharyngeal plexus and the recurrent laryngeal nerve branch of the vagus. There are no muscles that are directly attached to the cricoid cartilage that can move it forward. It requires approximation and fixation through the thyroid cartilage to the hyoid bone and their upward and forward displacement by a number suprahyoid and infrahyoid muscles including the geniohyoid muscles, which play a key role in the opening of the UES. Other muscles involved in hyolaryngeal elevation and therefore cricoid displacement are the mylohyoid, the anterior belly of the digastric, and the thyrohyoid. Key muscles that participate in UES opening in humans are innervated by cranial nerve V (superior hyoid movement) and spinal nerve C1 (anterior hyoid movement.
Q16. What is the neural deficit that is responsible for the swallowing difficulty in this patient?
The patient has evidence of bilateral motor paresis of all cranial nerves involved in swallowing, namely, V, VII, IX, X, XI, and XII, although the right side may be more affected than the left side. A traumatic hemorrhage in the region of the central pattern generator (CPG) of the medulla is the most likely site for this pattern of neural involvement and is consistent with the other neurologic findings showing cranial nerve V, VII, IX, X, and XII dysfunction, mild ataxia, and no spasticity. In the presence of such extensive motor damage, it is not possible to identify with certainty whether the sensory pathway and the swallowing center itself is damaged. The proximity of the nucleus tractus solitarius and the swallowing center to the motor nuclei of the affected cranial nerves makes it highly likely that they are also damaged.
Q17. What diagnostic and therapeutic options would you consider?
We would consider continuing strategies to heighten swallow sensitivity and enhancement of pharyngeal muscle strength with tongue protrusion during attempted swallow (Masako maneuver). However, techniques to enhance opening of the UES may be most useful at this time. These techniques may include the following: (1) Head turn to the right, as the patient has greater weakness on the right side and the head turn might increase the pull to open UES; however, during the study, head turn did not help open the UES and facilitate transit of barium. (2) Continue Shaker exercises. (3) Given the findings of this VFSS and the patient's intact cognition, biofeedback to enhance the patient's awareness of the movement and strength of the suprahyoid muscles would involve segmental surface electromyography (sEMG) of the suprahyoid muscles that the patient will observe his own muscle activity as he performs maneuvers to strengthen these muscles such as Mendelsohn's maneuver. (4) Because the thyrohyoid muscle is important in elevation of the larynx and its fixation of the hyoid bone, exercises such as variation in voice pitch including vocal function exercises and falsetto may also be considered. (5) Upper esophageal sphincter dilation may be useful if there is fibrotic stricture of the CP muscle. In this patient there was no evidence of stricture on endoscopy. (6) If lack of opening of the UES is caused by spasm of the CP muscle, intrasphinteric injection of Botox into the UES may be considered. Whether the CP muscle is paralyzed or in a state of spasm cannot be determined by these studies. A manometry of the upper sphincter would be useful in making this distinction, although the presence of paralysis of the pharyngeal muscles and the cranial nerve X paralysis suggest that the UES may also be paralyzed and hypotensive.
Q18. What is Botox? Is it likely to be useful in this case?
Botox (botulinum toxin A) is taken up by the motor nerve endings by receptor mediated endocytosis. Inside the nerve terminal, the active part of the toxin is released, which acts to degrade a protein called SNAP-25. SNAP-25 is critical for the release of the neurotransmitter acetylcholine. The action of the toxin lasts for several months. Therefore, injection of the toxin in the CP muscle would paralyze it for several months. Botox may be useful if the CP muscle is in a state of hypercontraction or spasm and if the spasm is responsible for the impaired opening of the UES. However, it would not be of value if the sphincter opening is impaired because of the paralysis of the dilator muscles (suprahyoid muscles) that open the sphincter by causing anterior displacement of the cricoid cartilage. In this patient, the VFSS shows lack of opening (lack of forward displacement of larynx or the cricoid cartilage), indicating that the suprahyoid muscles are impaired, particularly geniohyoid and thyrohyoid severely limiting anterior cricoid displacement.
Suggested Reading
Asoh R, Goyal RK. Manometry and electromyography of the upper esophageal sphincter in the opossum. Gastroenterology 1978;74(3):514–520.
Cook IJ. Cricopharyngeal function and dysfunction. Dysphagia 1993;8:244–251.
Crary MA. A direct intervention program for chronic neurogenic dysphagia secondary to brainstem stroke. Dysphagia 1995;10:6–18.
Crary MA. Surface electromyographic characteristics of swallowing in dysphagia secondary to brainstem stroke. Dysphagia 1997;12:180–187.
Dodds WJ, Taylor AJ, Stewart ET, Kern MK, Logemann JA, Cook IJ. Tipper and dipper types of swallows. AJR 1989;153:1197–1199.
Fujiu M, Logemann JA. Effect of a tongue holding maneuver on posterior pharyngeal wall movement during deglutition. Am J Speech Lang Pathol 1996;5:23–30.
Gates J, Hartnell GG, Gramigna GD. Videofluoroscopy and swallowing studies for neurologic disease: a primer. RadioGraphics 2006;26:e22. doi:10.1148/rg.e22. Published November 8, 2005.
Goyal RK, Martin SB, Shapiro J, Spechler SJ. The role of cricopharyngeus muscle in pharyngoesophageal disorders. Dysphagia 1993;8:252–258.
Huckabee ML, Cannito M. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: a retrospective evaluation. Dysphagia 1999;14:93–109.
Jean A. Brain stem control of swallowing: neural network and cellular mechanisms. Physiol Rev 2001;81:929–996.
Kahrilas PJ, Logemann JA, Krugler C, Flanagan E. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol 1991;260:G450–456.
Lazzara G, Lazzarus C, Logemann JA. The impact of thermal stimulation on the triggering of the swallowing reflex. Dysphagia 1986;1:73–77.
Lazzarus C, Logemann JA. Swallowing disorders in closed head trauma patients. Arch Phys Med Rehabil 1987;68:79–87.
Logemann JA. Evaluation and Treatment of Swallowing Disorders, 2nd ed. San Diego: College Hill, 1998.
Logemann JA. The role of exercise programs for dysphagia patients. Dysphagia 2005;20:139–140.
Martino R, Terrault N, Ezerzer F, Mikulis D, Diamant NE. Dysphagia in a patient with lateral medullary syndrome: insight into the central control of swallowing. Gastroenterology 2001;121:420–426.
Ohmae Y, Ogura M, Taraho T, Kitahara S, Inouye T. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol 1998;107:344–348.
Pommerenke WT. A study of the sensory areas eliciting a swallowing reflex. Am J Physiol 1927;81(1):36–41.
Rasley A, Logemann JA, Kahrilas PJ, Rademaker AW, Pauloski BR, Dodds WJ. Prevention of barium aspiration during videofluoroscopic swallowing studies: value of change in posture. AJR 1991;160:1005–1009.
Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc 2005;53(9):1483–1489.
Rosenbek JC, Roecker EB, Wood JL, Robbins J. Thermal application reduces the duration of stage transition after stroke. Dysphagia 1996;11:225–233.
Rosenbek JC, Robbins J, Willford WO, et al. Comparing treatment intensities for tactile-thermal application. Dysphagia 1998;131–9.
Shaker R, Easterling C, Kern M, et al. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology 2002;122(5):1314–1321.