Abstract
Purporse
To determine the associated balance of forces of the vitreofoveal interface in focal vitreomacular traction evolving to full-thickness macular hole (FTMH) and to link/explain the observed changes in the context of mathematical and physics models.
Patients and methods
This is a multicenter, prospective, and observational case series conducted at the Vitreoretinal Department of three different referral centers. Fellow eyes of patients with unilateral idiopathic FTMH were included. Eighty-nine patients were included in the analysis. The fellow normal eye of the study patients was imaged with spectral-domain optical coherence tomography. The main outcome measure was the optical-coherence-tomography-defined characteristics of the vitreofoveal interface and their analysis with mathematical and physics models at the end of follow-up period.
Results
Of the included 89 patients (66 women and 23 men; mean age±SD, 68.5 years±9.8), 10 (11.2%) developed FTMH at the fellow eye at the end of the follow-up period. We observed two types of vitreofoveal attachment. A V-shaped (cord-like) configuration and a U-shaped configuration. The eyes with the V-shaped attachment demonstrated initial structural changes in the outer foveal layers and the eyes with the U-shaped attachment showed inner morphological changes.
Conclusion
We hypothesize that the type (V- or U-shaped) of the vitreofoveal attachment may affect the type and location of the initial structural change leading to the formation of FTMH from the stage of the focal vitreomacular traction.
Similar content being viewed by others
Introduction
Although macular hole (MH) represents a well-known clinical entity, the features of the underlying pathophysiological mechanism of its early development are not yet completely understood. Since 1988, when Gass1 and Johnson and Gass2 proposed what later became the widely accepted classification of MH based on biomicroscopy, there have been several attempts on optical coherence tomography (OCT)-based detailed anatomical depiction of the individual MH stages and the associated balance of forces contributing to the evolvement between these different stages.
According to the previously mentioned classification,1, 2 the earliest ophthalmoscopic sign during the development of MH is a foveolar yellow spot, comprising the stage 1-A impending MH. This initial change, creating a clinically observed color alteration is believed to represent a foveolar detachment, a finding that is supported by Takahashi et al3 following their observation of detachment of the cone outer segment tip (COST) line on spectral-domain OCT (SD-OCT) in the early stages of stage 1-A MH.
Based on their hypothesis,3 this alteration may not have been visible in the pre SD-OCT era where the generally asserted theory about the stage 1 initial structural change was a foveal pseudocyst or horizontal splitting in the inner retinal layer comprising the stage 1-A MH. This may evolve into stage 1-B MH, whereby the posterior expansion of the pseudocyst creates a disruption of the outer retinal layer with an exposed retinal pigment epithelium and an intact roof.4, 5, 6, 7, 8, 9, 10, 11
This stage has been recently redefined as a vitreomacular traction that can be further subclassified by size of the adhesion into focal (≤1500 μm) or broad (>1500 μm).12
Several questions remain unanswered with regards to the initial processes and morphological characteristics of the foveal microstructure in stage 1-A MH. Does the early anatomical change occur in the inner or in the outer foveal layers and which is the factor that may influence this topographic relation? Is the vitreous traction primarily anteroposterior or tangential, and how this balance of forces may affect the type of the early structural change? Do the initial morphological changes need to follow a specific pattern or is it the configuration of the perifoveal posterior vitreous detachment that may explain the differences in the previously reported observations in the literature?
The purpose of this study is to determine the characteristics of the anatomical changes of the foveal microstructure in stage 1-A MHs in relation to the associated balance of forces of the vitreofoveal interface and to link/explain the observed changes in the context of mathematical and physics models.
Materials and methods
Our study is a multicenter, prospective, and observational case series. Patients with full-thickness macular hole (FTMH) in one eye were included in the study. Originally, 95 consecutive patients, were examined between December 2009 and March 2013. Eighty seven of them were available for review in a mean follow-up period of 2.5 years (range 18–38 months) and were followed up at 2 months’ intervals. All patients were confirmed to have a unilateral FTMH at the initial visit by dilated indirect slit lamp biomicroscopy and OCT. The fellow eye of these patients was imaged with SD-OCT and were included in this study. The included in the study patients were recruited by the vitreoretinal units of 2nd Department of Ophthalmology, University of Athens; 2nd Department of Ophthalmology, ‘Henry Dunant’ Hospital, Athens and Ophthalmic Institute ‘OMMA’, Athens. All procedures performed followed the tenets of the Declaration of Helsinki; the study was approved by the institutional review board and ethics committee of the Hospital. All patients provided informed consent for all examinations.
Posterior vitreous detachment was considered to be present if the Weiss ring was clinically apparent and/or SD-OCT provided evidence of PVD. Except from idiopathic FTMH, patients with any other co-existent vitreoretinal pathology (eg, epiretinal membrane) or any other fundus diseases in the study eye or history of ocular trauma, or surgery in either eye were excluded from the study.
All fellow eyes of patients with FTMH underwent complete baseline ocular examination, including measurement of BCVA, slit-lamp examination and SD-OCT (spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) imaging through dilated pupils. The horizontal B-scan images were extracted from six radial-lines 3 mm long scanned through the center of the fovea. For each patient, six radial scans 3-mm long were performed at equally spaced angular orientations centered on the foveola. We ascertained that the scan intersection point was consistent over time (spectralis OCT with 7 μm axial resolution). The angle (θ) of the vitreofoveal attachment was measured manually. The area (a) of vitreomacular adhesion was measured using the OCT callipers application (spectralis OCT; Heidelberg Engineering). Repeated scans were obtained at 2 months’ intervals by the same experienced ophthalmologist, and the patients were followed up for a mean period of 2.5 years.
Statistical and mathematical/physics analysis
Descriptive statistics were used for statistical representation of demographic characteristics. Standard triangle trigonometry was used for mathematical analysis of angle of attachment in relation to the vector force exerted. The stresses were calculated assuming a static half-infinite homogeneous elastic medium when analyzing the foveal deformation in correlation with the area of vitreofoveal attachment.
Results
Eighty-nine consecutive patients with FTMH (stages 2, 3, and 4) were examined (66 women and 23 men; mean age±SD, 68.5 years±9.8; range 49–78). The eye with MH underwent vitrectomy with internal limiting membrane peeling and injection of 12% C3F8 gas. The patients were asked to posture face-down for one week. Of them, 10 patients (11.2%) developed FTMH and 24 eyes demonstrated spontaneous resolution of the vitreomacular traction in the fellow eye at the end of the follow-up period.
The typical clinical appearance of vitreomacular traction (loss of foveal depression and central yellow spot) was seen in 7 of the 10 patients. In 7 of the 10 eyes, SD-OCT revealed an intrafoveolar split or pseudocyst occupying the inner part of the foveola at the initial examination. The remaining three eyes demonstrated a foveal detachment at the COST line similar to the one observed by Takahashi et al.3
With regards to the vitreofoveal interface we observed two types of attachment; a V-shaped configuration and a U-shaped configuration. The V-shaped configuration included a cord-like posterior hyloid shape with smaller area of attachment (80–280 μm, see Table 1) and steeper angle of attachment (40/45–80/70 degrees, (angle measured at nasal/temporal side of vitreofoveal adhesion)). The U-shaped configuration was characterized by a biconvex posterior hyloid shape with broader area of adhesion (180–600 μm, see Table 1) and an angle of attachment ranging between 15/20 and 45/45 degrees. The eyes with the V-shaped attachment demonstrated initial structural changes in the outer foveal layers (at the COST line) and the eyes with the U-shaped attachment showed inner morphological changes.
The 24 eyes that demonstrated spontaneous resolution of the vitreofoveal traction during the follow-up period were all in the category of V-shaped vitreofoveal attachment configuration.
All eyes, irrespective to the topographic location of the initial anatomical disruption, evolved to a full-thickness separation (either toward the inner or the outer layers according to the type of the initial change) with intact roof and subsequently to stages 2–4 MH.
Selected case presentation
Case 1
A 65-year-old man with mild metaphorphopsia, best corrected visual acuity 20/25 and foveolar yellow spot on biomicroscopy in his left eye, was recruited in our study.
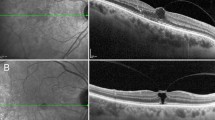
Baseline SD-OCT showed a V-shaped vitreofoveal traction and triangular foveolar detachment of the COST line (Figure 1a). The top of the detachment merged with the photoreceptor IS/OS layer. Two months later OCT images (Figure 1b) demonstrated the development of an intraretinal foveal pseudocyst, whereas the foveal detachment was enlarged and more elevated. The photoreceptor IS/OS layer was disrupted.
(a) Spectral-domain optical coherence tomography (SD-OCT) at baseline (selected case 1) showing a V-shaped vitreofoveal traction (horizontal white arrow) and a triangular foveolar detachment of the COST line (vertical arrow). The top of the detachment is merged with the photoreceptor IS/OS layer. The central foveal microanatomy is also depicted magnified. (b) SD-OCT of the same patient at 2 months’ follow-up examination demonstrating the development of an intraretinal foveal pseudocyst, whereas the foveal detachment was enlarged and more elevated. The photoreceptor IS/OS layer was disrupted. (c) SD-OCT of the same patient at four months’ follow-up showing an expansion and connection of the foveal detachment with the inner pseudocysts representing the development of a macular hole IB. (d) SD-OCT of the same patient at 6 months following the initial examination demonstrating the development of a full-thickness macular hole.
Four and six months after the first visit, SD-OCT (Figures 1c and d) showed that the foveal detachment was connected with the inner pseudocysts and FTMH development, respectively.
The vitreomacular traction was evident throughout the development of FTMH.
Case 2
A 62-year-old woman was recruited in the study. Baseline best corrected visual acuity was 20/32. Biomicroscopy showed a foveal pseudocyst with radial striae combined with a yellow spot. SD-OCT (Figure 2a) showed two intraretinal pseudocysts with a U-shaped vitreofoveal attachment; a larger one corresponding to the elevated foveal surface at the region of vitreofoveal traction and a smaller one next to it. A small elevation of the COST line was also detected.
(a) Spectral-domain optical coherence tomography (SD-OCT) at baseline (selected case 2) showing a U-shaped vitreofoveal traction (white horizontal arrow). Also, two inner intraretinal pseudocysts are observed; a larger one corresponding to the elevated foveal surface at the region of vitreofoveal traction and a smaller one next to it. A small elevation of the COST line was also detected (vertical white arrow). (b) SD-OCT of the same patient at 2 months’ follow-up examination revealing a foveolar detachment beneath the foveal cyst. (c) SD-OCT appearance of 8 months from baseline. The foveal pseudocyst as well as the foveal detachment were enlarged. OCT showed a large pseudocyst disrupting the outer layers of the retina, but with an intact roof. (d) One year from baseline. The intraretinal pseudocyst and the foveal detachment are connected, the roof had opened partially and a full-thickness macular hole developed.
Two months later (Figure 2b), SD-OCT showed a foveolar detachment beneath the foveal cyst. Six months later (Figure 2c), the foveal pseudocyst as well as the foveal detachment were enlarged. OCT showed a large pseudocyst disrupting the outer layers of the retina, but with an intact roof.
One year from baseline (Figure 2d) and as the vitreomacular traction was still evident, the intraretinal pseudocyst and the foveal detachment were connected, the roof had opened partially and FTMH developed.
Case 3
A 63-year-old woman was recruited in our study. She was complaining of decreased vision in her right eye (fellow eye). Best corrected visual acuity was 20/32. At baseline, SD-OCT examination (Figure 3a) demonstrated two intrafoveal splits within the inner foveal layers and a U-shaped vitreofoveal attachment. The photoreceptor inner segment and outer segment junction (IS/OS) layer was elevated. SD-OCT obtained 4 months (Figure 3b) later showed enlarged splits and a small triangularly shaped foveal detachment of the elevated photoreceptor IS/OS layer beneath the central fovea. A columnar structure between the splits beneath the central fovea and a small disruption in the photoreceptor IS/OS layer under the structure was also detected. In addition, there was a disruption of the roof of the pseudocyst on the right-hand side at the point of the vitreomacular adhesion.
(a) Spectral-domain optical coherence tomography (SD-OCT) at baseline (selected case 3) showing a U-shaped vitreofoveal traction (vertical white arrow). Also, two intrafoveal splits within the inner foveal layers are observed. In addition, a columnar structure between the splits is evident (horizontal white arrow). The photoreceptor inner segment and outer segment junction (IS/OS) layer was elevated. (b) SD-OCT of the same patient 4 months from baseline demonstrating enlarged splits and a small triangularly shaped foveal detachment of the elevated photoreceptor IS/OS layer beneath the central fovea (white arrow). A small disruption in the photoreceptor IS/OS layer under the structure was also detected. In addition, there was a disruption of the roof of the pseudocyst on the right hand side at the point of the vitreomacular adhesion. (c) Eight months after the initial visit the lower portion of the columnar structure was separated from the outer retinal layer and shortened anteriorly. A cone shaped structure under the inner roof is also visible (white arrow). The two intrafoveal splits and the foveal detachment are connected. (d) At 14 months’ follow-up, the conversion to a full- thickness macular hole is visible with a more extensive disruption of the inner roof.
Eight months after the initial visit (Figure 3c), the lower portion of the columnar structure was separated from the outer retinal layer and shortened anteriorly. A cone shaped structure under the inner roof is also visible. The two intrafoveal splits and the foveal detachment are connected. After 6 months, the conversion to a FTMH is visible with a more extensive disruption of the inner roof (Figure 3d).
Discussion
Many hypotheses have been formulated regarding the balance of forces contributing to MH formation. The initial reported concept of anteroposterior vitreous traction having a role in the early development of MH13 was later replaced by the Gass hypothesis1 of tangential traction of the vitreous cortex at the edges of the foveola, representing the primary force vector in MH formation. In the later years, new observations reinforced the role of anteroposterior traction during the evolvement between the early stages of MH.4, 14, 15, 16 In addition, the fact that approximately 50% of stage 1-A MHs (focal vitreomacular traction) resolve spontaneously after separation of the vitreofoveal adhesion and release of the traction3, 5, 17 highlights the role of traction as a primary causative characteristic over degenerative processes.18, 19
Furthermore, the other aspect of the exact underlying mechanism during the early stages of MH development is the type of the initial structural change that is caused by the balance of forces. Several reports19, 20, 21, 22, 23 based on OCT findings have described cystic changes or splits in the inner retinal layer as the first stage of MH formation. Hee et al21 presented a foveal cyst or a retinal split resulting from vitreomacular traction in the fellow eye of FTMH. Gaudric et al4 reported an intrafoveolar split and a large cyst in fellow eyes of MHs in their OCT study. They also proposed that vitreomacular traction at the inner retina is transmitted to the outer retina and induces deformation of the IS/OS photoreceptor junction line, and that this may be the first step in the opening of a hole. Few other cases suggest that MHs begin as shallow tractional foveal detachments.23
Moreover, Haouchine et al5 reported that the initial structural feature was an intrafoveolar spilt occupying the inner retinal layers. This commonly accepted hypothesis of an inner cyst (stage 1-A MH) progressing to stage 1-B MH following an outer segment expansion4, 5, 6, 7, 8, 9, 10, 11 was questioned by Takahashi et al,3, 24 suggesting that the foveolar detachment of the COST line seen on SD-OCT might represent the yellow spot seen clinically in the very early stages of MH.
In the present study 7 out of 10 patients who developed FTMH (in the initially normal fellow eye) demonstrated a clinically apparent yellow spot. SD-OCT revealed vitreofoveal attachment in all patients (the posterior hyaloid was partially detached over the posterior pole and remained strongly adherent only to the foveal center). Two configurations of vitreofoveal attachments were observed; a V-shaped (cord-like) configuration and a U-shaped configuration.
With regard to the topographic relation of the initial morphological change of the foveal microstructure, we observed an inner retinal split or foveal cyst in 7 out of 10 patients and further progression to the outer layers causing eventually FTMH. In the remaining three patients, foveolar detachment of the COST line was the first finding in the development of FTMH. Interestingly, there was a difference in the type of the configuration of vitreofoveal interface in relation to the location of the initial change. The eyes with the V-shaped attachment demonstrated initial structural changes in the outer foveal layers (at the COST line) and the eyes with the U-shaped attachment showed inner morphological changes.
We speculate that the initial foveal anatomical changes in a focal vitreomacular traction progressing to FTMH may vary according to the different types of anteroposterior vitreous traction. Based on mathematical and physics models, two main factors that may mechanically alter the balance of forces in the foveal microstructure are: (a) the angle of vitreofoveal attachment and (b) the area of attachment.
Angle of vitreofoveal attachment
The force vector of the vitreomacular traction can be expressed in two dimensions on the (x and y) plane. Moreover, angle ‘θ’ is the angle that the two-dimensional force vector makes with the x axis (Figure 4c).
Using right triangle trigonometry, Fx is adjacent to angle θ, Fy is opposite to angle θ. So, Fx=F × cosθ and Fy=F × sinθ.
In cases of U-shaped posterior hyaloid, the angle θ is smaller than π/4 and therefore cosθ>sinθ, which means that Fx>Fy. On the contrary, in V-shaped anteroposterior traction the angle is θ>π/4, and sinθ>cosθ, which means that Fy>Fx.
During the process of MH formation, when Fy>Fx, anteroposterior tractional forces at this axis may be transmitted to the foveal floor (outer segment) through the oblique focal attachment of the posterior hyaloid. Gass20, 25 proposed that Müller cell cone serves as a plug to bind together the receptor cells in the foveola having a significant role in early stage MH formation. On the contrary, when Fx>Fy, the tangential tractional forces may alter the inner layers of the retina initially.
Area of vitreofoveal attachment
Another factor that may have a role is that the type of initial changes in the outer or the inner layers of the retina may vary according to the distance between the sites of vitreomacular adhesion over the retina surface. In U-shaped cases of vitreomacular traction a ‘significant’ distance (a) between the two sites can be detected. On the contrary, in V-shaped configuration the distance between those sites is smaller (Figures 4a and b).
Quantitatively, the stress (σ) is expressed by the traction force (F) between adjacent parts of a material across an imaginary separating surface a, divided by the area of a (σ=F/a).26 The concept of the vitreomacular attachment being inversely correlated with macular morbidity and foveal deformation was also proposed by Spaide et al.27 The total force exerted on the fovea in relation to the distance (a) of the two different sites of vitreomacular traction is depicted in Figure 5a. The stresses were calculated assuming a static half-infinite homogeneous elastic medium.28
Graph (a) depicting the total force exerted on the fovea in relation to the distance (a) of the two different sites of vitreomacular traction. The stresses were calculated assuming a static half-infinite homogeneous elastic medium. The curve representing x=0 depicts the alterations of the traction force (F) concerning the inner layers of the retina, in relation to the distance (a) between the two sites of vitreomacular adhesion. On the other hand the curve x=ay depicts the changes of the traction (F) concerning the outer layers of the retina, in relation to the depth (y) and the distance (a) between the two sites of vitreomacular adhesion. Graph (b) depicting the correlation between the force exerted at different depths and the area of adhesion. As we can see, when the distance (a) between the two sites is very small, meaning that these two sites are very close, as in the cases of V-shaped vitreomacular traction, the changes will take place initially at the outer layers of the retina. Graph (c) depicting the correlation between the force exerted at different depths and the area of adhesion. When distance (a) is significant, as in U-shaped of vitreomacular configuration, then the first changes detected at the retina are more likely to occur at the inner layers of the retina.
The curve representing x=0 depicts the alterations of the traction force (F) concerning the inner layers of the retina, in relation to the distance (a) between the two sites of vitreomacular adhesion. On the other hand the curve x=ay depicts the changes of the traction (F) concerning the outer layers of the retina, in relation to the depth (y) and the distance (a) between the two sites of vitreomacular adhesion.
As we can see in Figure 5b,28 when the distance (a) between the two sites is very small, which means that these two sites are very close, as in cases of V-shaped configuration, the changes will take place initially at the outer layers of the retina. This is apparent in case 1 (Figure 1a).
On the contrary, when the distance (a) is significant (Figure 5c), as in U- shaped configuration, then the first changes detected are more likely to occur at the inner layers of the retina. This is something we observed in 7 out of 10 cases (Figure 2a).
Our study has an obvious limitation. The limited number of cases does not provide a justifiable statistical approach in support of our theoretical analysis. Of course, the fact that the majority of patients with early morphological changes have mild or few symptoms creates an additional difficulty in collecting and studying the exact pathophysiology of the early changes in MH formation.
We hypothesize that based on the previously mentioned rationale, the type (V- or U-shaped) of the vitreofoveal attachment may affect the type and location of the initial structural change leading to the formation of FTMH from the stage of the focal vitreomacular traction. Further studies are needed in order to confirm this hypothesis, quantify the features of the vitreofoveal adhesion, and possibly predict the pattern and severity of disruption of the foveal microstructure based on the configuration of the vitreomacular interface.

References
Gass JD . Idiopathic senile macular hole: its early stages and pathogenesis. Arch Ophthalmol 1988; 106: 629–639.
Johnson RN, Gass JD . Idiopathic macular holes: observations, stages of formation, and implications for surgical intervention. Ophthalmology 1988; 95: 917–924.
Takahashi A, Nagaoka T, Yoshida A . Stage 1-A macular hole: a prospective spectral-domain optical coherence tomography study. Retina 2011; 31 (1): 127–147.
Gaudric A, Haouchine B, Massin P, Paques M, Blain P, Erginay A . Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol 1999; 117: 744–751.
Haouchine B, Massin P, Gaudric A . Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical coherence tomography. Ophthalmology 2001; 108: 15–22.
Kishi S, Takahashi H . Three-dimensional observations of developing macular holes. Am J Ophthalmol 2000; 130: 65–75.
Niwa H, Terasaki H, Ito Y, Miyake Y . Macular hole development in fellow eyes of patients with unilateral macular hole. Am J Ophthalmol 2005; 140 (370): 375.
Azzolini C, Patelli F, Brancato R . Correlation between optical coherence tomography data and biomicroscopic interpretation of idiopathic macular hole. Am J Ophthalmol 2001; 132: 348–355.
la Cour M, Friis J . Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol Scand 2002; 80: 579–587.
Altaweel M, Ip M . Macular hole: improved understanding of pathogenesis, staging, and management based on optical coherence tomography. Semin Ophthalmol 2003; 18: 58–66.
Mirza RG, Johnson MW, Jampol LM . Optical coherence tomography use in evaluation of the vitreoretinal interface: a review. Surv Ophthalmol 2007; 52: 397–421.
Duker JS1, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013; 120: 2611–2619.
Avila MP, Jalkh AE, Murakami K, Trempe CL, Schepens CL . Biomicroscopic study of the vitreous in macular breaks. Ophthalmology 1983; 90: 1277–1283.
Kakehashi A, Schepens CL, Trempe CL . Vitreomacular observations, II: data on the pathogenesis of idiopathic macular breaks. Graefes Arch Clin Exp Ophthalmol 1996; 234: 425–433.
Akiba J, Quiroz MA, Trempe CL . Role of posterior vitreous detachment in idiopathic macular holes. Ophthalmology 1990; 97: 1610–1613.
Kim JW, Freeman WR, El-Haig W, Maguire AM, Arevalo JF, Azen SP . Baseline characteristics, natural history, and risk factors to progression in eyes with stage 2 macular holes: results from a prospective randomized clinical trial. Ophthalmology 1995; 102: 1818–1829.
de Bustros S, Vitrectomy for Prevention of Macular Hole Study Group. Vitrectomy for prevention of macular holes: results of a randomized multicenter clinical trial. Ophthalmology 1994; 101: 1055–1059.
Mc Donnell PJ, Fine SL, Hillis AI . Clinical features of idiopathic macular cysts and holes. Am J Ophthalmol 1982; 93: 777–786.
Morgan CM, Schatz H . Involutional macular thinning: a pre–macular hole condition. Ophthalmology 1986; 93: 153–161.
Gass JD . Müller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacular retinoschisis. Arch Ophthalmol 1999; 117: 821–823.
Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP et al. Optical coherence tomography of the human retina. Arch Ophthalmol 1995; 113: 325–332.
Kishi S, Kamei Y, Shimizu K . Tractional elevation of Henle’s fiber layer in idiopathic macular holes. Am J Ophthalmol 1995; 120: 486–496.
Asrani S, Zeimer R, Goldberg MF, Zou S . Serial optical sectioning of macular holes at different stages of development. Ophthalmology 1998; 105: 66–77.
Takahash A, Nagaoka T, Ishik S, Kameyama D, Yoshida A . Foveal anatomic changes in a progressing stage 1 macular hole documented by spectral-domain optical coherence tomography. Ophthalmology 2010; 117: 806–810.
Gass JD . Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol 1995; 119: 752–759.
Liu I-S Forces and moments. In: Liu I-S (ed). Continuum Mechanics. Springer: Berlin, Heidelberg; 2002. pp 41–50.
Spaide RF, Wong D, Fisher Y, Goldbaum M . Correlation of vitreous attachment and foveal deformation in early macular hole states. Am J Ophthalmol 2002; 133: 226–229.
Landau LD, Lifshitz EM . Theory of Elasticity (Course of Theoretical Physics). Pergamon Press, 1970.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Theodossiadis, G., Petrou, P., Eleftheriadou, M. et al. Focal vitreomacular traction: a prospective study of the evolution to macular hole: the mathematical approach. Eye 28, 1452–1460 (2014). https://doi.org/10.1038/eye.2014.223
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.223
This article is cited by
-
Morphologie des vitreoretinalen Übergangs am Partnerauge bei Patienten mit durchgreifendem Makulaforamen
Der Ophthalmologe (2018)
-
Idiopathic macular holes and direction of vitreomacular traction: structural changes and surgical outcomes
Eye (2017)
-
The design and validation of an optical coherence tomography-based classification system for focal vitreomacular traction
Eye (2016)
-
A retrospective cohort study in patients with tractional diseases of the vitreomacular interface (ReCoVit)
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)
-
Movement of the inner retina complex during the development of primary full-thickness macular holes: implications for hypotheses of pathogenesis
Graefe's Archive for Clinical and Experimental Ophthalmology (2015)