Abstract
Aim
To report the visual outcome of polypoidal choroidal vasculopathy receiving combined treatment with photodynamic therapy using Visudyne and intravitreal ranibizumab injections, and to analyze the predictive factors of visual outcome at 1 year post treatment.
Methods
Seventy-four consecutive patients with newly diagnosed polypoidal choroidal vasculopathy were treated with photodynamic therapy using Visudyne and three loading doses of intravitreal ranibizumab. The final visual outcome and polyp eradication rate at 1 year were reported. A stepwise regression model was used to estimate the baseline clinical factors predictive of better visual outcome and polyp eradication.
Results
Visual acuities at 12-months follow-up improved significantly compared with baseline from 0.828 logMAR to 0.728 logMAR (P=0.026). The mean foveal thickness decreased from 380±175 to 278±117 μm. In all 29.7% of eyes improved at least by 0.3 logMAR, and 55.4% remained stable in visual acuity with less than 0.3 logMAR change. Overall, 85% of eyes achieved at least stable vision, 20.2% (15/74) cases achieved polyp eradication on angiogram, and 60.8% (45/74) achieved polyp size reduction on angiogram at 1 year. Regarding predictive factors, the baseline visual acuity (P=0.003), no foveal involvement by abnormal choroidal vasculature (P<0.0001), absence of hard exudates (0.001) or subretinal fluid (<0.0001) are important clinical factors affecting the final visual outcome.
Conclusions
Combination therapy with photodynamic therapy using Visudyne and three loading doses of intravitreal ranibizumab injections resulted in 85% success rate on visual stabilization and 81% success rate in polypoidal lesion control.
Similar content being viewed by others
Introduction
Polypoidal choroidal vasculopathy (PCV) is a distinct macular degeneration entity characterized by a branching choroidal vascular network and polypoidal vascular dilatation.1 Although being associated with multiple recurrent serosanguineous detachments, PCV was reported to have better prognosis than age-related macular degeneration (AMD) as PCV is usually not linked to significant fibrous proliferation typical of end-stage neovascular AMD.2
Treatments for PCV have largely relied on photodynamic therapy (PDT) since the past decade, as PDT could eradicate abnormal choroidal polyps with up to 80% success rate.3, 4, 5, 6 Meanwhile, the role of anti-VEGFs in treating PCV has been ascertained by several prospective studies with successful outcomes.7, 8 Anti-VEGF works in a mechanism different from PDT; it reduces exudation related to vasculopathy and normalizes retinal thickness. As possible synergistic effect may result by combining the two methods, we therefore conducted this study to evaluate the efficacy of combination therapy with PDT using Visudyne and ranibizumab (Lucentis; Genentech, South San Francisco, CA, USA) in PCV cases.
Given the variability in clinical courses and visual outcomes in PCV cases, a risk stratification calculator based on predictive factors may help clinicians to determine the most suitable treatments and to counsel patients. Hence, a stepwise logistic regression analysis was adopted for building a risk-analysis profile for visual outcome and disease curative rate.
Patients and methods
This is a retrospective study of consecutive cases investigating the 1-year result of combination therapy with PDT and three loading doses of 0.5 mg ranibizumab injections followed by as-needed reinjection schedule. The recruitment period was from January 2010 to October 2012. Consecutive treatment-naive cases visiting our retinal clinic at the Prince of Wales Hospital, Chinese University of Hong Kong, were recruited.
Inclusion criteria included age >45 years old, newly diagnosed PCV with presence of classic polypoidal vasculature on indocyanine green angiography (ICGA). Recruited patients were those suffering from symptomatic PCV either directly related to polyp itself or secondary to exudative symptom regardless of the site of polyp occurrence; hence both extrafoveal and macular PCV were to be considered. Diagnosis of PCV was based on both fundal findings and ICGA findings. It was based on disease characteristics of elevated orange-red lesions (not resembling a PED, choroidal hemangioma, or subretinal blood) on clinical examination or characteristic polypoidal vasculature on ICGA.9 Exclusion criteria included previous history of AMD, history of PDT, a high myopia (of >6 D) and pre-existing macular scar.
All patients underwent a comprehensive ophthalmic examination before initiation of treatment, which included comprehensive slit-lamp biomicroscopy, fundus color photography (TRC50, Image Net, Topcon, Tokyo, Japan), ICGA (TRC-50IA, Image Net, Topcon), and spectral domain optical coherence tomography (OCT) (v. 1.6.4.0, Heidelberg Engineering, Heidelberg, Germany). The visual acuity was measured monthly. Best corrected visual acuity (BCVA) was measured in a well-illuminated room with optimal refractive correction, where the standard Snellen visual acuity chart was adopted and placed at a distance of 6 feet from the patient for measurement. BCVA was converted to the log minimum angle of resolution (logMAR) for analysis. OCT was performed at baseline, 3 months, 6 months and 1 year post initiation of treatment. Fluorescein angiography (FA) and ICGA were performed at baseline, 6 months and 12 months post treatment in all cases.
Patients were treated with a combination therapy of one session of standard PDT and three monthly doses of intravitreal ranibizumab. Regarding PDT application, all patients received a 6 mg/m2 infusion of verteporfin, followed by diode laser applied to the leaking choroidal polyps and abnormal vasculature guided by ICGA. A total light energy of 50 J/cm2 and light for 83 s were used to cover the entire lesion. The entire lesion was covered by laser spot with an additional 500 μm covering the borders on each side. Intravitreal ranibizumab injection was given simultaneously following uneventful PDT on the same day. Additional doses of ranibizumab were given in a monthly manner for up to three monthly loading doses.
Additional doses of intravitreal injections or repeated PDTs were administered in the event of persistent or recurrent disease.10, 11 Eligible patients for retreatments would be offered PDT as frequently as every 3 months. Repeated ranibizumab injections could be offered on a monthly basis. Fluorescein angiogram and indocyanine angiography were performed every 3 months in cases with clinical suspicion of persistent disease. Retreatment was considered when there was either angiographic or clinical evidence of persistent or recurrent disease. Angiographic evidence included persistent leakage on fluorescein angiogram or ICGA. Clinical evidence included increase in haemorrhage area on fundus photographs or increase in exudation documented on OCT. Persistent leakage directly underneath the fovea was also considered an indication for retreatment disregarding the extent of leak. Additional treatments were recommended as guided by the predominant pathology. PDT was suggested for prominent leaking polypoidal lesion, whereas ranibizumab injection was provided when significant exudations present. The number of additional ranibizumab injections was decided according to clinical response.
Outcome measures were divided into two categories, functional and anatomical success. Functional success was regarded as follows: good visual outcome (⩾0.3 logMAR gain), stabilized visual outcome (< 0.3 logMAR change in visual acuity) , and significant worsened visual outcome (⩾0.3 logMAR worse). BCVA results were converted to logarithm of the minimal angle of resolution (logMAR) value for statistical analysis. To assess the response on anatomic success, good response is regarded as reduced lipid exudation and reduced foveal thickness ⩽280 μm.
As PCV carried diversity in visual outcome, baseline ophthalmic clinical factors were collected to evaluate the relationship with the clinical course and outcome. The clinical factors collected include age, gender, size of the largest polyp, distance of the largest polyp to fovea, the greatest linear dimension (GLD) of the polyp, baseline visual acuity, size of subretinal blood, number of polyps, location of abnormal choroidal vasculature, area of treatment by PDT, and presence of the following: grape-like clustering of the polyp, subretinal blood >1 disc diameter, concurrent type-2 CNV, lipid exudation, subretinal fluid, and pigment epithelial detachment. The size of the largest polypoidal lesion, the GLD, and distance between the largest polyp and foveal center were measured by examining the ICGA images. Measurements were made by an electronic caliper via the software installed in an ICGA machine (TRC50; IMAGEnet; Topcon).
Patient characteristics were retrieved from corresponding medical records; information obtained included age at diagnosis, sex, and BCVA documented in decimal visual acuity charts. The baseline clinical factors retracted as predictors for analysis of long-term visual outcome were mentioned previously. The baseline clinical factors were adopted for univariable and multivariable univariate analysis against the outcome parameters. Post-treatment clinical information was not included in the regression analysis.
The difference between the baseline visual acuity and post-treatment visual acuity at 1 year has been compared using the paired Student t-test; a value of less than 0.05 was considered statistically significant. Stepwise logistic regression and linear regression were used for determining predictive factors for anatomical and visual outcomes, respectively, with statistical tests of P-value <0.05 being considered significant. Statistical analysis was performed with BM SPSS 18.0 software for Windows (SPSS/IBM Corporation, Chicago, IL, USA).
Results
Treatment outcomes
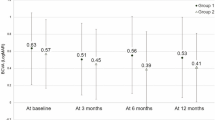
Table 1 shows the demographics of 74 eyes of 74 patients and the baseline ophthalmic clinical findings. There were 51 males (68%) and 24 females (32%). The visual acuities 1 year after the combination therapy improved significantly from the baseline (P=0.026; paired Student t-test). The mean baseline visual acuity was 0.828 logMAR±0.54 SD, and the mean post-treatment visual acuity was 0.728logMAR±0.54SD. The mean improvement was 0.1 logMAR unit. The mean baseline foveal thickness was 380±175 μm, and the mean post-treatment foveal thickness measured at 6 months post-treatment was 278±117 μm.
Visual outcome at 1 year in our center was reported as follows. The visual acuity improved at least 0.3 logMAR unit at 1 year after the combination therapy in 22 eyes (29.7%) and remained stable in 41 eyes (55.4%) with less than 0.3 logMAR change in VA. In 11 eyes (14.9%), visual acuity dropped more than 0.3 logMAR at 1 year after treatment. Overall, the visual acuity remained unchanged or improved in 85.1% of eyes (63/74). Regarding anatomical outcome, good outcome was observed in 56 eyes (75.7%) at 6 months post initiation of treatment. FA performed 12 months post initiation of PDT treatment showed polypoidal lesions completely resolved in 15 eyes (20.2%), and decreased in size or number in 45 eyes (60.8%). Overall, 81% of cases achieved angiographic success with evidence of polypoidal eradication or reduction at the sixth month.
Evaluation of treatment-resistant cases and adverse outcomes
The initial three monthly loading doses of ranibizumab were injected into 74 eyes. Nine eyes were found to suffer from clinically evident persistent leakage from polypoidal lesion with significant exudations, three eyes (4.1%) received two additional reinjections, and six eyes (8.1%) received extra three injections. Among these cases, two eyes (3%) underwent one additional PDT session 3 months apart from the first PDT. Only 1 case out of 74 cases (1.4%) suffered from subretinal hemorrhage of larger than 1 disc diameter after combination therapy throughout the 1-year follow-up period.
Predictive factors for visual outcome
Predictive factors for final visual outcome were estimated by regression modelling. Univariate analysis showed that baseline characteristics such as younger age, better baseline visual acuity, larger distance between the largest polyp and fovea, smaller size of the largest polyp, no foveal inovlvement by polypoidal lesion, and absence of subretinal fluid or hard exudates at the macula correlated significantly with better visual acuity at 1 year (Tables 2a and b). Backward stepwise multivariable regression analysis for linear variables showed that baseline visual acuity (regression coefficient=0.496; adjusted R2=0.470; P=0.003) and location of choroidal vasculature (0.467; adjusted R2=0.560; P=0.008) were two independent factors most significantly related to the visual outcome at 1 year. (Table 3) In summary, the important predictor factors for visual outcomes included better baseline visual acuity, farther location of polypoidal vasculature from fovea, no foveal involvement by the polypoidal lesions, and absence of hard exudates or subretinal fluid at the macula.
Predictive factors in determining successful polyp eradication
Univariate logistic regression showed that several factors were related to the poor response of polypoidal lesions treatment. Bigger size of the largest polyp (coefficient=−0.004; P=0.007), increased number of polyps (coefficient=−0.300; P=0.043), and presence of clustering grape-like polypoidal lesions (coefficient=−1.656; P=0.005) were shown to correlate significantly with poor angiographic response (Table 4). In 56 eyes with good response of polyp eradication, the mean VA at 1 year post treatment was 0.536 logMAR (Snellen equivalent to 20/70)±0.13, which was significantly better than the mean VA in poor polypoidal lesion response group of 0.992 logMAR (Snellen equivalent to 20/200)±0.536 (P=0.028; Student’s t-test). In the good angiographic response group, 19 eyes (34%) had visual improvement >0.3 logMAR; compared with only three eyes (17%) in the poor angiographic response group.
Discussion
Verteporfin PDT can achieve polyp regression in 80–95% of cases.3, 4, 5, 6 It is well recognized for the treatment of PCV through ‘thrombosis’ of the abnormal vessels. On the other hand, anti-VEGFs act through a different mechanism by restoring the normal retinal thickness. Rapid restoration of edematous retina could potentiate the final visual outcome. Our study proved the synergistic effect of the combination regime by achieving 85% rate in visual stabilization and 81% rate in polyp eradication at 1 year.
Anti-VEGF was reported to have a lower efficacy in eradicating the polypoidal choroidal structure in eyes with PCV, given the persistence of polypoidal lesions or branching vascular networks in previous reports using anti-VEGF as sole treatment.12, 13, 14, 15, 16 However, the high anatomic conversion rate by verteporfin PDT is not without costs. PDT may cause putative damages to the PRE by causing choriocapillary hypoperfusion and retinal pigment epithelium atrophy. The damage by PDT was demonstrated by reduction of retinal function as documented on multifocal electroretinogaphy.17 Recent researchers adopted enhanced depth imaging OCT in PCV examination, showing reduction of choroidal thickness after PDT application.18 A subnormal choroidal thickness secondary to choroidal vasculature thrombosis may hinder the visual outcome.19 Hence, PDT is seemingly not desirable for multiple recurrence cases.
A direct comparison of PDT with ranibizumab treatment was carried out in a randomized, multicenter trial of 93 treatment-naive cases in the Laptop study.20 In all 17% of eyes in the PDT arm achieved visual gain compared with 30.4% in the ranibizumab arm. The superiority of ranibizumab in PCV in terms of visual outcome was proved, but the effectiveness of combined treatment was not evaluated.20 The EVEREST study was the only randomized study comparing the three arms for PCV, including ranibizumab, PDT, and combined therapy.15 The study concluded that PDT was more effective than ranibizumab in causing regression of polyps, with the mean gain in visual acuity being similar among the three groups.15 Despite being a well-conducted randomized trial, the sample size was relatively small, with only 17 cases per arm. Our study supplemented information on the efficacy of the combination regime with a relatively large series of 74 cases, which further supports the use of combination therapy by achieving good stabilization of VA.
The long-term visual outcome in the eyes with PCV depends on the recurrence rate and size of atrophic changes that result from persistent lesions. Wakabayashi et al21 showed that regression of polypoidal lesions early after PDT treatment could be up to 94%; however, 42% of cases showed evidence of recurrence with an enlarged vascular network at a mean follow-up of 19.2 months. It is still an educated assumption that polyp clearance reduces disease recurrence. Our study concluded some predictors may indicate poor polypoidal conversion and perhaps increased disease recurrences. However, we did not evaluate the direct linkage between anatomic clearances of polyp and recurrence rate.
PDT combined with anti-VEGF therapy is currently recommended for treatment of PCV and was reported to have good visual outcome in patients suffering from PCV.22, 23, 24, 25 However, relatively few studies have investigated the visual prognostic factors following PCV treatments,26, 27, 28, 29, 30 especially the predictive factors following combined therapy. Some of the clinical factors such as age, baseline best-corrected visual acuity and size of haemorrhage, and presence of PED were reported to govern the final visual outcome.26 Recently, the baseline polyp size and the location of polyps were also proven to affect the long-term visual acuity in a retrospective cohort.31 However, these results were rather inconsistent. Our study could help bridge the knowledge gap by a relatively large sample size and uniform follow-up schedules.31 In our study, the most significant good predictive factors for visual outcomes included better baseline visual acuity, farther location of polypoidal vasculature from the fovea, no foveal involvement by the polypoidal lesions, and absence of hard exudates or subretinal fluid at the macula. Compared with the existing studies, presence of PED or large area of subretinal blood was not necessarily related to final visual acuity. The authors considered PED or subretinal blood as an associated feature in PCV but not a valuable predictor.32 Instead, presence of hard exudates or subretinal fluid at macula signifies poor visual outcome. Any information regarding the polyp nature, such as the polyp size, the number of polyps, and clustering of polyps, was regarded as information related to polyp eradication ease rather than final visual outcome. The authors suggested a possible indirect pathway linking the persistence of polyps to recurrence of disease and eventually affecting long-term visual prognosis.31
Mori et al33 reported the good predictors for PCV patients receiving PDT as sole treatment in 181 patients. Attention was drawn to the size of the GLD for abnormal vasculature. However, the size of GLD did not correlate well with the final visual outcomes in our series. Instead, the location of lesions did matter; any active subfoveal or juxtafoveal polypoidal lesions may result in more profound damage to photoreceptors irrespective of the size of lesion. Our result on predictive factors is useful in foreseeing a patient’s visual outcome at presentation solely by judging the location and extent of dilated vascular networks. In addition, the mean distance from the largest polyp to the fovea in our series was found to be 1224 μm, reflecting most cases with polypoidal lesions in close proximity to the fovea. Careful judgment is needed in such cases; the authors suggest choosing anti-VEGF agents instead of PDT in treating foveal or juxtafoveal lesions to avoid direct RPE damage. This important information may serve as a compass to ophthalmic surgeons whenever they need a better comprehension of the possible treatment benefit and risk via a tactical choice of the best treatment regimen.
The current study has limitations due to its retrospective nature and a lack of data regarding comparison arms. A randomized study or a control group is preferred in direct comparison of anti-VEGF agents, PDT, and a combination of both. This study did not look into the relationship between polyp conversion and recurrence rate. Future studies with longer follow-up for information regarding disease recurrence are preferred. Regarding the strength, it provides an insight into the predictive factor profiles in patients receiving combined therapy. Our study further supports the efficacy of adopting the combination regime in PCV cases and is valuable for the understanding of PCV as it is one of the largest cohort study evaluating the treatment outcome of PCV. The authors also suggest risk stratification in choosing individualized treatment for PCV patients.
In conclusion, combined therapy by both PDT and ranibizumab injections results in good visual outcome at 1 year. Combination therapy could achieve good disease control angiographically of up to 85% cases. The authors concluded that both anti-VEGF agents (at least three loading doses) and PDT using Visudyne are essential in treating PCVs. However, it is also important to exert clinical judgment in choosing monotherapy based on individualized disease characteristics.

References
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B . Idiopathic polypoidal choroidal vasculopathy (IPCV). 1990. Retina 2012; 32 (Suppl 1): 1–8.
Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA . Polypoidal choroidal vasculopathy. Surv Ophthalmol 2004; 49 (1): 25–37.
Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology 2004; 111 (8): 1576–1584.
Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 2008; 115 (1): 141–146.
Silva RM, Figueira J, Cachulo ML, Duarte L, Faria de Abreu JR, Cunha-Vaz JG . Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefe's Arch Clin Exp Ophthalmol 2005; 243 (10): 973–979.
Otani A, Sasahara M, Yodoi Y, Aikawa H, Tamura H, Tsujikawa A et al. Indocyanine green angiography: guided photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2007; 144 (1): 7–14.
Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K et al. Factors predictive of outcomes 1 year after 3 monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Retina 2013; 33: 1949–1958.
Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K et al. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am J Ophthalmol 2012; 154 (1): 117–24 e1.
Yuzawa M, Mori R, Kawamura A . The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol 2005; 89 (5): 602–607.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007; 143 (4): 566–583.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 2009; 148 (1): 43–58 e1.
Cho HJ, Kim JW, Lee DW, Cho SW, Kim CG . Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye (Lond) 2012; 26 (3): 426–433.
Lai TY, Lee GK, Luk FO, Lam DS . Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina 2011; 31 (8): 1581–1588.
Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 2008; 92 (1): 70–73.
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012; 32 (8): 1453–1464.
Wakabayashi T, Gomi F, Sawa M, Tsujikawa M, Nishida K . Intravitreal bevacizumab for exudative branching vascular networks in polypoidal choroidal vasculopathy. Br J Ophthalmol 2012; 96 (3): 394–399.
Lai TY, Chan WM, Lam DS . Transient reduction in retinal function revealed by multifocal electroretinogram after photodynamic therapy. Am J Ophthalmol 2004; 137 (5): 826–833.
Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T . Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2011; 151 (4): 594–603 e1.
Ho M, Liu DT, Chan VC, Lam DS . Choroidal thickness measurement in myopic eyes by enhanced depth optical coherence tomography. Ophthalmology 2013; 120 (9): 1909–1914.
Oishi A, Kojima H, Mandai M, Honda S, Matsuoka T, Oh H et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month laptop study results. Am J Ophthalmol 2013; 156: 644–651.
Wakabayashi T, Gomi F, Sawa M, Tsujikawa M, Tano Y . Marked vascular changes of polypoidal choroidal vasculopathy after photodynamic therapy. Br J Ophthalmol 2008; 92 (7): 936–940.
Saito M, Iida T, Kano M . Combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Retina 2012; 32 (7): 1272–1279.
Saito M, Iida T, Kano M, Itagaki K . Two-year results of combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 2013; 251 (9): 2099–2110.
Sato T, Kishi S, Matsumoto H, Mukai R . Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for polypoidal choroidal vasculopathy. Am J Ophthalmol 2010; 149 (6): 947–54 e1.
Tomita K, Tsujikawa A, Yamashiro K, Ooto S, Tamura H, Otani A et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy combined with intravitreal injections of ranibizumab. Am J Ophthalmol 2012; 153 (1): 68–80 e1.
Hikichi T, Ohtsuka H, Higuchi M, Matsushita T, Ariga H, Kosaka S et al. Factors predictive of visual acuity outcomes 1 year after photodynamic therapy in Japanese patients with polypoidal choroidal vasculopathy. Retina 2011; 31 (5): 857–865.
Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S . Predictive factors of resolved retinal fluid after intravitreal ranibizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 2011; 95 (11): 1555–1559.
Saito M, Iida T, Nagayama D . Photodynamic therapy with verteporfin for age-related macular degeneration or polypoidal choroidal vasculopathy: comparison of the presence of serous retinal pigment epithelial detachment. Br J Ophthalmol 2008; 92 (12): 1642–1647.
Tsujikawa A, Ojima Y, Yamashiro K, Nakata I, Ooto S, Tamura H et al. Association of lesion size and visual prognosis to polypoidal choroidal vasculopathy. Am J Ophthalmol 2011; 151 (6): 961–72 e1.
Yamashiro K, Tomita K, Tsujikawa A, Nakata I, Akagi-Kurashige Y, Miyake M et al. Factors associated with the response of age-related macular degeneration to intravitreal ranibizumab treatment. Am J Ophthalmol 2012; 154 (1): 125–136.
Kang HM, Koh HJ, Lee CS, Lee SC . Combined photodynamic therapy with intravitreal bevacizumab injections for polypoidal choroidal vasculopathy: long-term visual outcome. Am J Ophthalmol 2014; 157 (3): 598–606 e1.
Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K et al. Factors predictive of outcomes 1 year after 3 monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Retina 2013; 33 (9): 1949–1958.
Mori R, Yuzawa M, Lee Z, Haruyama M, Akaza E . Factors influencing visual outcome of polypoidal choroidal vasculopathy 1 year after photodynamic therapy. Graefes Arch Clin Exp Ophthalmol 2010; 248 (9): 1233–1239.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author Contributions
All authors listed here are qualified for authorship. This is to certify all the authors gained credit by fulfilling substantial contributions to the conception and design, acquisition and analysis of data, drafting and revising the article, and approving the version to be published.
Rights and permissions
About this article
Cite this article
Ho, M., Lo, E., Young, A. et al. Outcome of polypoidal choroidal vasculopathy at 1 year by combined therapy of photodynamic therapy with ranibizumab and predictive factors governing the outcome. Eye 28, 1469–1476 (2014). https://doi.org/10.1038/eye.2014.222
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.222
This article is cited by
-
Real-world outcomes of combined therapy of photodynamic therapy with anti-vascular endothelial growth factor for polypoidal choroidal vasculopathy
Eye (2022)
-
The polyp regression rate and treatment prognosis of different interventions for polypoidal choroidal vasculopathy: a systematic review and meta-analysis
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Treatment of polypoidal choroidal vasculopathy by photodynamic therapy, aflibercept and dexamethasone triple therapy
Scientific Reports (2016)
-
Factors influencing the outcome of polypoidal choroidal vasculopathy following combined treatment with photodynamic therapy and intravitreal ranibizumab
Eye (2015)
-
Reply to ‘Factors influencing the outcome of polypoidal choroidal vasculopathy following combined treatment with photodynamic therapy and intravitreal ranibizumab’
Eye (2015)