Abstract
Purpose
The purpose of this study was to present an association between diabetic macular oedema (DME) and vitreoretinal interface abnormalities using 3D spectral domain optical coherence tomography (SD-OCT).
Methods
In a retrospective study, charts and SD-OCT of consecutive patients with diffuse or focal DME were reviewed. Only one randomly chosen eye per patient with DME was included, and eyes that had another retinopathy that could affect the study analysis or that underwent vitreoretinal surgery were excluded.
Results
Out of 58 eyes (58 patients) with DME, 11 eyes (19.0%) had vitreofoveal traction (Group A), either unifocally (n=6) or multifocally; that is, associated with additional extrafoveal traction site(s). Group B comprised 20 eyes (34.5%) that had sole extrafoveal vitreous traction, at either retinal and/or papillary sites. In each, the retinal oedema underlying extrafoveal traction was in continuum in at least one site with that at the central macula, as verified by the macular maps, thus presented as diffuse macular oedema. In Group C, 13 eyes (22.4%) had an epiretinal membrane (ERM), 1.5 × 3 mm to ⩾6 × 6 mm in size that overlaid diffuse oedematous macula. Group D included 14 eyes (24.1%) that had neither vitreous traction nor ERM; 12 (20.7%) of them had DME secondary to leaking microaneurysms with or without leaking capillary beds, and the remaining two had leakage from non-microaneurysms sources.
Conclusions
DME was detected by the SD-OCT to be associated with sole extrafoveal vitreous traction in one-third of the patients. Further studies are required to evaluate the clinical consequences of these observations.
Similar content being viewed by others
Introduction
Macular oedema is a major cause of visual loss in patients with diabetic retinopathy. Diabetic macular oedema (DME) is frequently categorised into focal and diffuse types. The focal type of DME often relates to well-defined discrete areas of leakage from microaneurysms (MAs) as detected by fluorescein angiography (FA), and is responsive to focal argon laser photocoagulation treatment.1 The diffuse DME is characterised by generalised areas of leakage in the area centralis.2, 3 However, the pathogenesis of diffuse DME is, for the most part, poorly understood. As a consequence, its treatment by laser grid photocoagulation or by the intravitreal administration of medications is unsatisfactory and/or only temporarily efficacious in many cases.1, 2, 4, 5
For decades there has been a continuous effort to identify and understand the pathogenesis of the diffuse type of DME. Using time-domain optical coherence tomography (OCT-1, -2 or -3), researchers have determined four main factors to be associated with the diffuse type of DME:
-
1
Vitreomacular traction.6 Its prevalence in the larger series on DME ranged between 4% of eyes (6/140, described as a taut thickened posterior hyaloid),7 13.4% (25/186 eyes)8 and 25% (12/48 eyes; described as vitreoretinal adhesions).9 Pars plana vitrectomy (PPV) was generally found to be beneficial in these cases.6, 10, 11
-
2
Extrafoveal vitreous traction, manifesting as extrafoveal vitreoretinal8 or vitreopapillary12 traction. Researchers have found both vitreofoveal (13.4%) and extrafoveal (10.8%) traction to be prevalent in a total of 24.2% (45/186 eyes) of all eyes with DME.8
-
3
Studies noted a prevalence of epiretinal membrane (ERM) that ranged between 27%13 and 34%9 in eyes with DME. Few authors have identified the DME as having been predominantly developed by the ERM mechanical traction. They reported improvement in best-corrected visual acuity (BCVA) and/or level of the macular oedema following PPV and peeling of the ERM in eyes with DME.14, 15 None of the studies that used time-domain OCTs referred to the extent of the ERM, especially whether it was minimal or widespread.
-
4
In other cases, the pathogenesis of diffuse DME is ambiguous and is often related to vasoactive factors that are presumed to contribute to its development.16 These include vascular endothelial growth factor (VEGF),17 protein kinase C,18 nitrous oxide,19 erythropoietin,20 and more, as presented in a recent review.21 All these factors may have a role in the increase of vascular permeability, arterial dilation, increased macular blood flow,22 and the establishment of macular oedema.
The limitations of the time-domain OCT instruments in the detection of vitreous traction membranes8 and ERMs23 led to the purpose of this study, to use 3D spectral domain OCT (SD-OCT) to determine the prevalence of vitreous traction membranes and ERMs in eyes with DME. To the best of our knowledge (search through Entrez PubMed), this is the first report using SD-OCT in relation to this issue.
Patients and methods
In a retrospective study, we reviewed the charts of consecutive patients who were selected from our SD-OCT 1000 (Topcon Corp., Tokyo, Japan) data. Clinical ophthalmologic examination included Snellen BCVA, slit-lamp examination, and fundus examination. On biomicroscopy, all eyes had macular thickening and/or cystoid spaces involving the centre of the macula. OCT scans of all eyes were performed through a dilated pupil by two trained examiners. If both eyes per person had DME, we used only one eye, which was randomly chosen, for analysis.
3D data sets centred on the fovea (6 × 6 mm) were obtained for each patient. As a rule, 3D data sets were also centred on the optic nerve head (ONH) in association with the central macula, and were obtained using a raster scan programme of 8.2 mm (horizontal) × 3 mm (vertical) × 1.7 mm (axial). Volumetric rendering of the data set was performed using image-processing software within the SD-OCT for 3D image reconstruction. The SD-OCT characteristics were described elsewhere.24 Data, including the B- and 3D modes, could be evaluated later by examining the recorded video.
Cystoid spaces at the foveal region, as detected by OCT, were defined as intraretinal round hyporeflective lacunae with well-defined boundaries and hyper-reflective septa separating the cystoid-like cavities. Diffuse DME was identified by OCT as an ill-defined and widespread hyporeflective increased retinal thickness that often attains the appearance of sponge-like cavities.25, 26 When fovea lost its shape of depression and attained a cystoid elevation, it was termed ‘peaked fovea’. Macular oedema that is peaked at the fovea would present central cystoid macular oedema. Because the anatomical boundaries of the elevated oedematous macula cannot usually be precisely delineated, we designated vitreous traction at the central macula as ‘vitreofoveal traction’.8 Evidence of traction required vitreous adherence to the retina or ONH associated with tissue elevation and deformity at the traction site; that is, the shape of the inner retina at the exact site of traction changed its angle and thus was typically thicker than that of the adjoining oedematous retinal tissue. ‘Vitreous adherence’ (without traction) relates to eyes in which the attachment of the vitreous is not associated with any deformity at that site. Vitreous attachment was plainly detected in most eyes using the 3D mode because the entire width of the scanned vitreous membranes and retinal surface are exposed. Cases where the existence of tissue elevation and traction was uncertain could often be confirmed by using the near-histology appearance as detected by the B-mode. Vitreous traction at one site (unifocal) away from the oedematous central macula was designated as ‘extrafoveal traction’, either at the area centralis between the vascular arcades (‘extrafoveal vitreoretinal’ traction) or at the ONH (vitreopapillary traction). When there were two or more traction sites, traction was designated as ‘multifocal’. The 6-mm macular maps and the false-colour maps provided quantitative and qualitative information on the thickness of the retinal tissue at the site in question and were quantitatively compared with those of the normal controls.
ERM is generally presented as being either globally adherent and/or focally adherent.27 The diagnosis of globally adherent ERM is based on a difference in the red (false colour) brightness of the surface tissue that is more easily noticeable by the SD-OCT than by the time-domain OCT.28 In comparison with the time-domain OCT, the SD-OCT could make approximate measurements of the ERM area, as depicted in the running movie of its B-mode.
The study was limited to eyes that had diffuse or focal DME, as verified by the SD-OCT. Based on our normative database (n=43 eyes of 43 patients; mean age 64±12 years, range 29–89), the mean thickness of the central sub-field was 235±18 μm. The fovea could be designated as oedematous when cystoid spaces were located at its site, even if the tissue was not abnormally thickened, due to the fact that it could thin secondary to atrophy or lamellar hole formation. Exclusion criteria were eyes that had another retinopathy that could affect the data analysis; eyes that had undergone vitreoretinal surgery; eyes that had sole extrafoveal traction but whose underlying retinal oedema was not in continuum with the central macular oedema; eyes, for which FA was not available, that were not associated with vitreoretinal interface abnormality; and eyes for which scans were not of sufficiently high quality for a proper diagnosis. Calculations on the BCVA were converted to a logarithm of the minimum angle of resolution. Comparison between both the rates of PDR and earlier argon laser photocoagulation in the macula and/or panretinal photocoagulation (PRP) in the various groups was analysed statistically by using the Fisher's exact test.
Research adhered to the tenets of the Declaration of Helsinki Principles, and the approval of the institutional ethics committee was obtained.
Results
A total of 77 eyes with proliferative or non-proliferative diabetic retinopathy of 58 consecutive patients were found to have DME. Only one patient's eye (n=58), which was randomly chosen, was included in the study analysis (Table 1). Six other eyes were excluded from the study because of the unavailability of FA for eyes that were not associated with vitreoretinal interface abnormalities (n=2), the presence of an extrafoveal traction whose underlying retinal oedema did not appear to be in continuum with the central macular oedema (n=1), or because of inadequate quality of the OCT scans for a proper diagnosis or analysis (n=3). The vitreoretinal interface abnormalities that have been associated with DME are divided into four groups. Demographic patients’ data, type of diabetic retinopathy, and earlier treatment in the analysed eyes are described per each group in Table 1. Altogether, 39 (67.2%) patients were originated from North Africa or the Middle East and the other 19 (32.8%) patients were Caucasians (Table 2 and Supplementary Tables 1 and 2).
Group A includes 11 eyes of 11 patients (19.0% of 58) with vitreofoveal traction (Tables 1 and 2). The vitreofoveal traction was apparent either unifocally at the central macula (n=6 eyes; 54.5% of Group A or 10.3% of all 58 eyes) or in a multifocal manner, that is, associated with additional extrafoveal traction site(s) (n=5 eyes; Figure 1 and Supplementary video clip 1). In one (9.1% of Group A, or 1.7% of 58 eyes) of these eyes (no. 15) the vitreofoveal traction presented by the 3D mode as a vitreoschisis: the partially detached posterior hyaloid was seen to turn posteriorly in its central site in a sloped appearance, and while approaching the central macula, the cortical vitreous attained a ‘sliced’ appearance in which delicate vitreous strands were observed to adjoin the central macula. The shape of the central macular oedema was peaked in five eyes (45.4% of the group), combined peaked and diffuse in three eyes (27.3%), and diffusely thickened (without central peak) in the remaining three eyes (27.3%). Fluorescein angiography was performed in six eyes (54.5% of Group A): leakage was apparent at the central macular site in all six eyes, which ranged from well-defined focal area that presented by the OCT as relatively shallow, distinct oedema, and up to widespread leakage that appeared in the OCT scans as diffuse oedema. All 11 eyes of Group A had incomplete PVD that was associated with the vitreous traction.
A 66-year-old patient with diabetic macular oedema (no. 5, Table 2). (a) The clinical picture shows non-proliferative diabetic retinopathy and macular oedema. (b) The SD-OCT B-scan discloses an extrafoveal vitreous traction site that is located temporo-inferiorly to the central macula. The traction site is marked by a white vertical line of the OCT software, and this same site is also marked in the clinical picture by the green cross of the OCT software. Diffuse macular oedema and submacular fluid (asterisk) are detected. (c) The 3D mode reveals vitreous traction membranes in multifocal sites, at the fovea (arrow no. 1), at the vicinity of the ONH (arrow no. 2), and at the extrafoveal retina (arrow no. 3). The macula is thickened and submacular fluid is detected (asterisk).
Group B comprised 20 eyes (34.5%) that had sole extrafoveal vitreous traction. Extrafoveal traction was often (8 eyes; 40.0% of Group B, or 13.8% of all 58 eyes) apparent in multifocal sites, that is at extrafoveal vitreoretinal (Figure 2, Supplementary Figure 1 and Supplementary video clips 2 and 3) and/or vitreopapillary site(s) (Figure 3). An underlying retinal oedema was apparent at each traction site, which was in continuum in at least one site per eye with the oedema at the central macular area, as manifested by the B-mode and the 6-mm macular maps, and so presented as diffuse macular oedema. The oedema was either merely diffusely thickened (n=10; 50% of Group B) or combined with peaked fovea (n=10; 50%). Fluorescein angiography was performed in 14 eyes (70%) of Group B: In all of these 14 eyes (100% of Group B or 24.1% of all 58 eyes), at least one traction site per eye, either retinal and/or ONH, was associated with leakage from underlying capillaries or MAs. In the remaining eye, OCT revealed retinal oedema at the traction site, but leakage could not be identified. All eyes of Group B had incomplete PVD that was associated with the vitreous traction.
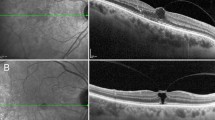
A 63-year-old patient with diabetic macular oedema (no. 23, Table 2) associated with extrafoveal vitreoretinal traction without vitreofoveal traction. (a and b) Two traction sites (1 and 2), one in each of the B-mode scans. The underlying retinal oedema in each is in continuum with the central macular oedema, attaining a diffuse oedema pattern. (c and d) The distribution of the macular oedema is seen in the two maps, the qualitative one with the false colours (c) and the quantitative map (d); the oedema is prominent only between the traction sites (1 and 2) and up to the central macular area. (e) The 3D mode shows the two vitreous traction sites (1 and 2) away from the fovea (fovea is marked by the vertical line).
A 50-year-old patient with diabetic macular oedema (no. 16, Table 2). (a and b) The B-mode and 3D SD-OCT mode, respectively, present vitreopapillary traction by the posterior hyaloid associated with macular oedema. (c) The 6-mm macular map reveals thickening of the macula in all quadrants, more in the nasal site. N, nasal; T, temporal.
Altogether (Tables 1 and 2), of the 58 eyes with DME, 31 eyes (53.5%) were associated with vitreofoveal (19.0%) and/or extrafoveal (34.5%) vitreous traction. Of these 31 eyes, we detected unifocal traction site in 19 eyes (61.3%) and multifocal traction sites in the other 12 eyes (38.7%). In 7 of the 31 eyes (22.6% of Group A+B or 12.1 % of all eyes) with vitreous traction, an associated ERM was also apparent (Table 2). The ERM area ranged between <1 × 1 mm and ⩾6 × 6 mm in size.
For the 13 eyes (22.4%) in Group C, a sole ERM without accompanied vitreous traction was detected (Table 1 and Supplementary Table 1). The ERM presented in 10 eyes (76.9% of Group C or 17.2% of the 58 eyes) as focally adherent in most of its area, and in the rest of the area as globally adherent; for the remaining 3 eyes, the ERM was globally adherent in most of its area and focally adherent in the rest of the area. The area of the ERM ranged in each eye from 4.5 mm2 (1.5 × 3 mm) to ⩾36 mm2 (6 × 6 mm), that is, the entire 6 × 6 mm scanned central field or more. The ERM mostly overlaid the diffuse oedematous macula in each eye (Supplementary video clip 4). The shape of the macula was diffusely thickened in 10 eyes, and combined diffuse with peaked fovea in the remaining 3 eyes. Fluorescein angiography was performed in nine eyes (69.2% of the group) and each eye presented with widespread leakage from MAs and dilated capillaries.
Group D contains the 14 remaining eyes (24.1%), which had neither vitreous traction nor ERM (Table 1 and Supplementary Table 2). Of these eyes, 12 (85.7% of Group D or 20.7% of all eyes) had DME that appeared secondary to leaking MAs with or without leaking dilated capillary beds. The other two eyes had leakage from non-MA vascular sites. Of all 14 eyes, well-defined single focal area of leakage was apparent by FA in 3 eyes, which appeared as distinct area of focal macular oedema in 1 eye and in a more diffuse pattern with indistinct borders in 2 eyes. Widespread leakage was apparent in the other 11 eyes, all of which appeared by OCT as diffusely thickened macula. Of all 14 eyes, the central macula was merely peaked in 2 eyes, combined peaked and diffuse in 4 eyes and diffusely thickened in the remaining 8 eyes. Macular ischemia was detected in 8 eyes (57.1% of Group D).
There was no statistically significant difference (P>0.05) among the relatively small groups in regards to either the rate of proliferative diabetic retinopathy or earlier argon laser photocoagulation at the macula and/or PRP.
Discussion
With the aid of the 3D SD-OCT, we found that extrafoveal vitreous traction site(s) without accompanying vitreofoveal traction was apparent in 34.5% of the 58 consecutive patients with DME. The retinal oedema that underlined the extrafoveal traction site of each eye was in continuum in at least one of the traction sites with the oedema at the central macular area, thus presenting as diffuse macular oedema. Vitreofoveal traction, with or without added extrafoveal traction was detected in other 19.0% of the eyes. Comparison of the prevalence of vitreous traction at the central macula with earlier, larger series, reveals a similar or increased prevalence when higher resolution and improved technology is used: A prevalence of 4% (6/140) of taut, thickened posterior hyaloid, a subjective sign, in eyes with DME was reported with the use of OCT-1;7 however, the authors note that this 4% frequency is lower than might have been expected from the literature, in which 21% of individuals described to have undergone vitrectomy for DME had done so in the context of a taut, thickened posterior hyaloid. In other studies, a prevalence of vitreous traction was found in 13.4% (25/186) of eyes with DME using OCT-2,8 and 25% (12/48) of vitreous adherence with OCT-1 and OCT-3 following at least one laser treatment.9 It would be important to determine whether the traction membranes initiated secondary to the vasculopathy process at the retina or not.
In this study, 34.5% of eyes had sole extrafoveal traction site(s) without traction at the vitreofoveal site. In comparison, the single study that described extrafoveal traction using the OCT-2 line group programme, found only 10.8% of eyes with DME to have extrafoveal traction.8 The considerable difference in the prevalence of extrafoveal vitreous traction between these two studies could be related to several factors:
-
1
The difficulty, while using the time-domain OCT, in trying to ‘seize’ and unambiguously verifying a point of vitreoretinal adherence, even if both relevant vitreoretinal tissues were very close together.8 Such was the case, despite frequent changes in direction of the scan line, focus, and length (shorter scan increases resolution) while moving manually from one retinal point to the other. In contrast, the B-mode of the SD-OCT scans each point at the studied area (Supplementary video clip 2); the 3D mode presents the whole scanned retinal area as well as the area of the scanned vitreous membrane that is adjacent to the retina or ONH (Supplementary video clips 1 and 3), so adherence site can be plainly detected by the SD-OCT even if it is very tiny.
-
2
A search for an extrafoveal vitreous traction site by the OCT-2 line group programme is an unavoidable time-consuming task to definitively prove vitreous adherence.8 Undertaking such a task often results in patient fatigue, gradual corneal drying, and progressive reduction in the quality of the scans, which could result in the omission of points of interest. By contrast, the SD-OCT scans the entire field of interest, usually as 6 × 6 mm followed by 3 × 8 mm, and in less than 3.7 s each, allowing for an improved point-by-point evaluation.
-
3
The higher resolution of the SD-OCT (6 μm) in comparison with the OCT-2 (∼12 μm).
-
4
The inability to re-observe more than a few scans that were saved when using the time-domain OCT-2. In contrast, use of the SD-OCT enables the re-evaluation of the whole scanned field and findings by using the saved movies.
PPV was found to be efficacious in the presence of vitreomacular traction in eyes with DME.10, 11 Following the controversy concerning whether PPV is beneficial in eyes in which vitreous traction is not detected,29, 30, 31 La Heij et al.32 found that surgical outcomes were significantly better in eyes that had not had previous laser treatment(s) than in eyes that had undergone laser photocoagulation for DME. The authors suggested that the reduced benefit of PPV in ‘non-tractional’ macular oedema was due to repeated laser applications and the increased endurance of the oedema before the PPV took place. Furthermore, each of the aforementioned studies that addressed the surgical outcome for ‘non-tractional’ DME29, 30, 31 was evaluated by using a time-domain OCT and an extrafoveal vitreous traction was neither reported nor ruled out. In addition, an explanation for the success of surgery in the non-tractional cases has not been put forward, although some believe it could be related to the removal of the vitreal growth factors that are in the vicinity of the retina.33 It is possible that our current findings might be of relevance to this controversy.
A solitary, relatively broad ERM, between 4.5 mm2 (1.5 × 3 mm) and ⩾36 mm2 (6 × 6 mm) in size, without associated vitreous traction, was detected in 13 eyes (22.4% of all eyes with DME; Table 1). In each case, diffuse macular oedema underlay the ERM site throughout most of its width. In another seven eyes, ERMs of various sizes were associated with a vitreous traction (Table 2). This gives a total of 34.5% of eyes (20/58) with ERM. A similar prevalence of ERM has been reported in other studies using time-domain OCT.13, 14 Naturally, in the studies that used time-domain OCT instruments the ERM size was not measured. In contrast, the running movie of the SD-OCT throughout the whole scanned field enabled a rough measurement of the ERM area. Whether ERM size has a role in the surgical outcome following its removal in eyes with DME is not yet known.
Following efficacious excision of the ERM in DME, Gandorfer et al.14 examined the specimen and found that the vitreous was attached to the macula. This condition is in contrast with the ERM of nondiabetic patients, where the ERM is associated with posterior vitreous detachment in most cases.34 Researchers have also reported that VEGF and interleukin-6 are localised to cells of vascular and avascular ERMs in patients with diabetic retinopathy.13, 35 In that regard, Antonetti et al.36 reported that increased levels of VEGF in the vitreous decrease the levels of occludin, a membrane-spanning tight junction protein, which in turn could alter the structure of the retinal endothelial junction, thereby increasing vasopermeability and macular oedema. Therefore, resolution of macular oedema in DME after removal of the cortical vitreous and ERM may not only be related to release of tractional forces but may also be caused by eliminating factors that enhance vasopermeability.14 Further studies are required to confirm the beneficial outcome of dissecting ERM that overlies a diffusely oedematous macula in DME.
Limitations of the study are related to its retrospective design and the relatively small groups. However, the current series finds that DME is associated with (1) a sole extrafoveal vitreous traction in one-third of the eyes and (2) vitreofoveal traction, extrafoveal traction, or ERM in most of the eyes. The detection of vitreous traction and ERM in diffuse DME may potentially identify patients that would benefit from early surgical intervention, with or without subsequent treatment(s).

References
Early Treatment Diabetic Retinopathy Study Research Group R. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS No. 19. Arch Ophthalmol 1995; 113: 1144–1155.
Lee CM, Olk RJ . Modified grid laser photocoagulation for diffuse diabetic macular edema: long term visual results. Ophthalmology 1991; 98: 1594–1602.
Gandorfer A . Diffuse diabetic macular edema: pathology and implications for surgery. Dev Ophthalmol 2007; 39: 88–95.
Jonas JB, Kreissig I, Sofker A, Degenring RF . Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol 2003; 121: 57–61.
Shinura M, Nakazawa T, Yasuda K, Shiono T, Iida T, Sakamoto T et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol 2008; 145: 854–861.
Lewis H, Abrams GW, Blumenkranz MS, Campo RV . Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology 1992; 99: 753–759.
Thomas D, Bunce C, Moorman C, Laidlaw AH . Frequency and associations of a taut thickened posterior hyaloid, partial vitreomacular separation and subretinal fluid in patients with diabetic macular edema. Retina 2005; 25: 883–888.
Ophir A, Trevino A, Fatum S . Extrafoveal vitreous traction associated with diabetic diffuse macular oedema. Eye 2010; 24: 347–353.
Ghazi NG, Ciralsky JB, Shah SM, Campochiaro PA, Haller JA . Optical coherence tomography findings in persistent diabetic macular edema: the vitreomacular interface. Am J Ophthalmol 2007; 144: 747–754.
Massin P, Duguid G, Erginay A, Haouchine B, Gaudric A . Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol 2003; 135: 169–177.
Song SJ, Sohn JH, Park KH . Evaluation of the efficacy of vitrectomy for persistent diabetic macular edema and associated factors predicting outcome. Korean J Ophthalmol 2007; 21: 146–150.
Karatas M, Ramirez JA, Ophir A . Diabetic vitreopapillary traction and macular oedema. Eye 2005; 19: 676–682.
Yamamoto T, Akabane N, Takeuchi S . Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol 2001; 132: 369–377.
Gandorfer A, Rohleder M, Grosselfinger S, Haritoglou C, Ulbig M, Kampik A . Epiretinal pathology of diffuse diabetic macular edema associated with vitreomacular traction. Am J Ophthalmol 2005; 139: 638–652.
Sakimoto S, Saito Y, Nakata K, Sakamoto Y, Tatebayashi M . Surgical outcomes of epiretinal membrane removal after successful pars plana vitrectomy for retinal diseases. Jpn J Ophthalmol 2008; 52: 227–230.
Dvorak HF, Brown LF, Detmar M, Dvorak AM . Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995; 146: 1029–1039.
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331: 1480–1487.
Nagpala PG, Malik AB, Vuong PT, Lum H . Protein kinase C beta 1 overexpression augments phorbol ester-induced increase in endothelial permeability. J Cell Physiol 1996; 166: 249–255.
Oku H, Kida T, Sugiyama T, Hamada J, Sato B, Ikeda T . Possible involvement of endothelin-1 and nitric oxide in the pathogenesis of proliferative diabetic retinopathy. Retina 2001; 21: 647–651.
Hernández C, Fonollosa A, García-Ramírez M, Higuera M, Catalán R, Miralles A et al. Erythropoietin is expressed in the human retina and it is highly elevated in the vitreous fluid of patients with diabetic macular edema. Diabetes Care 2006; 29: 2028–2033.
Bhagat N, Grigorian RA, Tutela A, Zarbin MA . Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol 2009; 54: 1–32.
Park JH, Woo SJ, Ha YJ, Yu HG . Effect of vitrectomy on macular microcirculation in patients with diffuse diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol 2009; 247: 1009–1017.
Falker-Radler CI, Glittenberg C, Binder S . Spectral domain high-definition optical coherence tomography in patients undergoing epiretinal membrane surgery. Ophthalmic Surg Lasers Imaging 2009; 40: 270–276.
Hangai M, Ojima Y, Gotoh N, Inoue R, Yasuno Y, Makita S et al. Three-dimensional imaging of macular holes with high-speed optical coherence tomography. Ophthalmology 2007; 114: 763–773.
Catier A, Tadayoni R, Paques M, Erginay A, Haouchine B, Gaudric A et al. Characterization of macular edema from various etiologies by optical coherence tomography. Am J Ophthalmol 2005; 140: 200–206.
Yamamoto S, Yamamoto T, Hayashi M, Takeuchi S . Morphological and functional analyses of diabetic macular oedema by optical coherence tomography and multifocal electroretinograms. Graefes Arch Clin Exp Ophthalmol 2001; 239: 96–101.
Wilkins JR, Puliafito CA, Hee MR, Duker JS, Reichel E, Coker JG et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology 1996; 103: 2142–2151.
Koizumi H, Spaide RF, Fisher YL, Freund KB, Klancnik Jr JM, Yannuzzi LA . Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. Am J Ophthalmol 2008; 145: 509–517.
Dillinger P, Mester U . Vitrectomy with removal of the internal limiting membrane in chronic diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 2004; 242: 630–637.
Figueroa MS, Contreras I, Noval S . Surgical and anatomical outcomes of pars plana vitrectomy for diffuse nontractional diabetic macular edema. Retina 2008; 28: 420–426.
Patel JI, Hykin PG, Schadt M, Luong V, Bunce C, Fitzke F et al. Diabetic macular oedema: pilot randomised trial of pars plana vitrectomy vs. macular argon photocoagulation. Eye 2006; 20: 873–881.
La Heij EC, Hendrikse F, Kessels AG, Derhaag PJ . Vitrectomy results in diabetic macular edema without evident vitreomacular traction. Graefes Arch Clin Exp Ophthalmol 2001; 239: 264–270.
Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol 2006; 142: 961–969.
Appiah AP, Hirose T, Kado M . A review of 324 cases of idiopathic premacular gliosis. Am J Ophthalmol 1988; 106: 533–535.
Chen YS, Hackett SF, Schoenfeld CL, Vinores MA, Vinores SA, Campochiaro PA . Localisation of vascular endothelial growth factor and its receptors to cells of vascular and avascular epiretinal membranes. Br J Ophthalmol 1997; 81: 919–926.
Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW . Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 1998; 47: 1953–1959.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Eye website
Supplementary information
Rights and permissions
About this article
Cite this article
Ophir, A., Martinez, M., Mosqueda, P. et al. Vitreous traction and epiretinal membranes in diabetic macular oedema using spectral-domain optical coherence tomography. Eye 24, 1545–1553 (2010). https://doi.org/10.1038/eye.2010.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.80
Keywords
This article is cited by
-
Mid-term safety and effectiveness of macular peeling one month after intravitreal dexamethasone implant for tractional diabetic macular edema
Scientific Reports (2023)
-
Factors associated with diabetic macular edema in patients with proliferative diabetic retinopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Vitrectomy for diabetic macular edema and the relevance of external limiting membrane
BMC Ophthalmology (2021)
-
En face image-based classification of diabetic macular edema using swept source optical coherence tomography
Scientific Reports (2021)
-
Incidence and treatment outcomes of secondary epiretinal membrane following intravitreal injection for diabetic macular edema
Scientific Reports (2020)