Abstract
Purpose
To study the efficacy of de-epithelialized amniotic membrane (AM) graft (AmbioDry, Okto Ophtho Inc., Costa Mesa, CA, USA) as an adjunctive therapy after primary pterygium excision in comparison to standard conjunctival autograft.
Methods
A retrospective review of 23 eyes of 22 patients receiving pterygium excision followed by AM transplantation was performed. The results were compared retrospectively with 40 eyes of 36 patients receiving conjunctival autograft after pterygium excision. All patients were Hispanic. Recurrence was defined as regrowth of fibrovascular tissue over the corneoscleral limbus onto clear cornea in the area of previous pterygium excision.
Results
The pterygium recurrence rates after AM graft and conjunctival autograft were 35 and 25%, respectively. There was no significant difference in recurrence rate between the two groups (P=0.56). The mean follow-up period was 5.9±2.4 months. No major complications were noted in either group.
Conclusion
This study provides preliminary evidence that de-epithelialized AM graft is as effective as conjunctival autograft in preventing pterygium recurrence in this Southern California Hispanic population.
Similar content being viewed by others
Introduction
Pterygia are a worldwide cause of cosmetic complaints, chronic irritative symptoms, and decreased vision secondary to induced astigmatism or growth over the visual axis.1 Although histologically benign, pterygia have a high tendency to recur after simple, bare sclera excision with reported incidences as great as 88%.2 Various adjuvant therapies have been used to reduce recurrence after excision. Conjunctival autografting is a well-accepted procedure and has proven to be both safe and effective in reducing pterygium recurrence with reported rates as low as 2%.3
Recently, the use of preserved human amniotic membrane (HAM) has been advocated for the management of many ocular surface disorders such as covering defects created after excision of conjunctival intraepithelial neoplasia and tumours,4, 5 scars, and sympblephara associated with surgical trauma after pterygium excision, chemical burns, ocular-cicatricial pemphigoid, and Stevens–Johnson syndrome.4, 5, 6 The benefits of using HAM in pterygium surgery were first described by Prabhasawat et al.7 Since then, multiple studies have reported the successful use of HAM with or without conjunctival autografting in eyes with primary and recurrent pterygia.8, 9, 10 To date, however, most of the clinical experience with the use of HAM in pterygium surgery and its many other indications has been with the use of the cryopreserved, epithelialized HAM (Amniograft, Biotissue Inc., Miami, FL, USA). In this study, we report the clinical outcome of HAM transplantation after pterygium excision using de-epithelialized, electron beam-sterilized amniotic membrane (AM) (Ambiodry, Okto Ophtho Inc., Costa Mesa, CA, USA). These results are compared retrospectively with conjunctival autograft after primary pterygium excision.
Materials and methods
Patients
Approval for conducting this project was obtained from the Institutional Review Board of University of California, Irvine (HS No 2003-3126).
A retrospective review of 23 eyes of 22 patients who underwent excision of primary pterygia followed by de-epithelialized HAM transplantation from October 2002 to March 2003 was performed. The results with respect to age, gender, race, recurrence rates, and complications were compared retrospectively with those of 40 eyes of 36 patients receiving excision of primary pterygia followed by conjunctival autograft during the same time period. Preoperatively, slit-lamp photographs were obtained. All patients resided in Southern California and were Hispanic. All procedures were performed by the same surgeon (AKF).
Surgical technique
After povidone–iodine preparation of the eyelids and conjunctival cul de sac, sterile draping, and instillation of topical anaesthetic (tetracaine 1%), a subconjunctival injection of 2% lidocaine with epinephrine 1 : 100 000 was given beneath the pterygium head. The pterygium head was removed from the cornea by blunt dissection using forceps and Wescott scissors. All Tenon's and subconjunctival fibrous tissue was excised upward and downward toward the fornices and medially towards but not reaching the caruncle. The corneal surface was then smoothed using either a knife or diamond-encrusted burr.
For AM grafts, the preserved dry AM (Ambiodry, Okto Ophtho Inc., Costa Mesa, CA, USA) was cut to the same size as the conjunctival defect. The graft was placed at the basement membrane side facing upward. The membrane was fixed to the recipient's conjunctival edge by interrupted 9-0 Vicryl sutures making sure that the edges were imbricated beneath the host conjunctiva with episcleral bites. For conjunctival autografts, tissue from the superior bulbar region was dissected free of Tenons capsule and secured with interrupted 9-0 Vicryl sutures. The remaining superior conjunctiva was re-approximated to the limbus using two interrupted 9-0 Vicryl sutures. Neomycin, polymixin b sulphate, and dexamethasone ointment (Maxitrol, Alcon Laboratories Inc., Fort Worth, TX, USA) was placed in all eyes immediately postoperatively and a pressure patch applied.
After surgery, all patients received ciprofloxacin 0.3% (Ciloxan, Alcon Laboratories Inc., Fort Worth, TX, USA) eye drops four times a day for 1 week depending on the presence of corneal epithelial defect and prednisolone acetate 1% (Pred Forte, Allergan, Irvine, CA, USA) four times daily, tapered over 2 months.
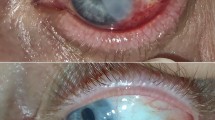
Follow-up visits were scheduled with the surgeon for postoperative day 1, 1 week, 1 month, 3 months, and 6 months. Photographs were obtained at each visit or at the time of recurrence (Figure 1). Invasion of any fibrovascular tissue past the corneoscleral limbus onto clear cornea in the area of previous pterygium excision constituted treatment failure and the patient was discharged from the study.
Statistical analysis
Statistical analysis was carried out with the Student's t-test (two-tailed), Fisher's exact test, and χ2-test. Statistical significance was considered when the P-value was <0.05.
Results
Comparison of demographic data between the two groups revealed a larger number of eyes and a larger number of patients in the conjuctival autograft group. All other preoperative characteristics were similar between the two groups (Table 1). All patients were Hispanic. Data from 63 pterygium excisions was analysed. The preoperative pterygium extended an average of 2.7±0.7 mm past the corneoscleral limbus onto the cornea. Sixty-one pterygia were nasal and two were temporal.
In the conjunctival autograft group, recurrence occurred in 10 eyes (25%). The mean time to recurrence was 2.3±0.9 months (range, 1–4 months) (Table 2). In the AM graft group, recurrence occurred in eight eyes (35%). The mean time to recurrence was 3.2±1.0 months (range, 1.0–5.5 months) (Table 2). There was no significant difference in the recurrence rate among the two groups (P=0.56) (Table 2). The mean time to recurrence for the conjunctival autograft group was significantly shorter than the AM group (P=0.005). The mean age of all patients with recurrence (31.3±9.8) was lower than that of patients without recurrence (37.0±12.1), although this trend was not statistically significant (P=0.08, Table 2).
Minor complications such as pyogenic granuloma (4.3%), epithelial defect (4.3%), and dellen (4.3%) were noted in eyes receiving AM grafts. These rates were similar to those for pyogenic granuloma (2.5%) and dellen (2.5%) in eyes receiving conjunctival autograft (P=0.66). All complications resolved.
Discussion
The primary concern after pterygium surgery is recurrence. It is not clear why recurrence occurs, but it is believed that postoperative inflammation, hyperproliferation of conjunctival fibroblasts,11 and overexpression of certain proinflammatory cytokines and metalloproteinases12 could be causative factors. A number of surgical techniques have been devised in an attempt to reduce recurrence after pterygium excision. In this study, we retrospectively compare two accepted techniques: HAM transplantation and conjunctival autograft.
The exact mechanism by which AM confers it beneficial effects in reconstruction of damaged ocular surface is not well understood. Studies suggest the beneficial features of promoting epithelialization and inhibiting inflammation and fibrosis.13 AM may provide a basement membrane rich in various growth factors and matrix proteins,14 which promotes epithelial cell migration,15 adhesion,16 and differentiation.17, 18 AM is also believed to have anti-inflammatory19, 20 and antifibrotic effects.21, 22
Pterygium recurrence rates following HAM transplantation have been reported as low as 3.0% following primary pterygium excision.8 All published clinical experience to date with the use of HAM in pterygium surgery has been with the use of epithelium-containing, cryopreserved HAM stored on a nitrocellulose substrate. At the time of surgery, the cryopreserved HAM is removed from the storage medium after thawing and cut to the same size to cover the defect. It is then sutured into place with the epithelial basement membrane side facing up.
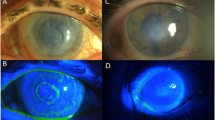
In this study, we describe the use of HAM that is denuded of epithelium. While maintaining the epithelial-promoting, anti-inflammatory and antifibrotic features of the frozen HAM, we believe that this HAM has several advantages. The graft is free-standing, thus eliminating the extra time and step needed to separate the graft form nitrocellulose substrate. As it is stored dry, there is no need for cold storage or thawing. The graft can be applied to the surgical site while dry, activated with saline, and sutured into place. Also, the grid pattern in the tissue allows quick visual differentiation of the stromal side, which is convex, from the basement membrane side, which is concave and indented (Figure 2). There is also a suggestion that HAM denuded of epithelium is a better substrate for the culture of corneal epithelial cells than AM with epithelium,23 although this finding cannot be directly applied to the conjunctival epithelium.
We compare our results following dry HAM with those of conjunctival autografting, which is by many24, 25, 26 considered the ‘benchmark’ to which other techniques should be compared. Our recurrence rate following de-epithelialized HAM transplantation was 35%, which is not significantly different than the recurrence rate of 25% following conjunctival autograft. These rates are higher than recent previous reports.5, 7, 9 Possible reasons for this variability include differences in technique and study population. Solomon et al6 describe an overall recurrence rate of 5.6% after extensive removal of pterygia and subconjunctival tissue, including the semilunar fold in some and postoperative injection of subconjuncitval steroids with signs of increasing inflammatory activity. Ma et al9 described recurrence rates of 5.4 and 3.8% for conjunctival autograft and AM graft, respectively, in a Chinese population in Taiwan with a mean age of 56.7 years. Just as we found, in a study population of young Hispanics, using a technique similar to ours, Chen et al2 reported a recurrence rate of 39% following conjunctival autograft. They found age to correlate significantly with recurrence. We also believe age to be an important factor in recurrence after pterygium excision. The mean age of patients with recurrences in our study was 31.3 years old, which is younger than those without recurrence (37.0 years), however not significant. We believe that race may also play a role in the extent and severity of postoperative inflammation and associated recurrence rates,2, 27 although we cannot compare racial groups in our solely Hispanic study population.
The short follow-up period in our study is obviously a drawback. It is documented that recurrences can occur up to 1 year postoperatively,28 but most recurrences occur within 4–6 months.2, 8, 9 Time to recurrence varies greatly from one study to another. Chen et al2 report a mean time to recurrence of 4.8 months after conjunctival autograft. Prabahasawat et al7 report a time to recurrence of 4.1±3.7 months after HAM transplantation. We report comparable results. Our time to recurrence is 3.2±1.0 and 2.3±0.9 months after AM transplant and conjunctival autograft, respectively. Also, although there was no clinically significant difference in the recurrence rates among the two groups, there is a trend toward an increased rate of recurrence with HAM (35%) in comparison with CAG (25%). With longer follow-up, it is possible for this difference to reach clinical significance.
This study has weaknesses inherent to any retrospective study. As all surgeries were performed by one surgeon during the same time period, there is potential for bias on the part of the surgeon as to which method to use for the different patients. The preoperative characteristics of the pterygia and the patients, however, do not differ among two groups (Table 1), minimizing the possibility of significant bias in choosing a surgical technique.
In summary, we report the first study comparing de-epithelialized HAM with conjunctival autograft after pterygium excision. In our study, dry de-epithelialized HAM was as effective as conjunctival autograft for pterygia in our predominantly young, Hispanic Southern California population. Neither technique was associated with any severe complications after a limited follow-up period. Although conjunctival autograft is considered to be an optimal adjunctive technique following pterygium removal, HAM grafting, particularly dry HAM with its greater ease of storage and handling, might successfully be used as an alternative in the surgical management of pterygia. In cases where a conjunctival autograft is not possible, where a large area needs to be restored or the conjunctiva is scarred or needs to be preserved for future glaucoma-filtering surgery, dry de-epithelialized HAM can provide similar results. Given the limitations of this study and overall limited experience with the dry de-epithelialized HAM, further investigation of the use of this material for pterygium surgery and other indications in ocular surface reconstruction is needed.
References
Oldenburg JB, Garbus F, McDonnell JM, McDonnell PJ . Conjunctival pterygia: mechanism of corneal topographic changes. Cornea 1990; 9: 200–204.
Chen PP, Ariyasu RG, Kaza V, LaBree LD, McDonnell PJ . A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am J Ophthalmol 1995; 120: 151–160.
Tan DT, Chee SP, Dear KB, Lim AS . Effect of pterygium morphology on pteryium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol 1997; 115: 1235–1240.
Tesavibul N, Prabhasawat P . Amniotic membrane transplantation in conjunctival surface reconstruction: a prospective study. Invest Ophthalmol Vis Sci 2001; 42: 8270.
Tseng SC, Prabhasawat P, Lee SH . Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol 1997; 124: 765–774.
Solomon A, Espana EM, Tseng SCG . Amniotic membrane transplantation for reconstruction of the conjunctival fornices. Ophthalmology 2003; 110: 93–100.
Prabhasawat P, Barton K, Burkett G, Tseng SC . Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 1997; 104: 974–985.
Solomon A, Pires RF, Tseng SC . Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology 2001; 108: 449–460.
Ma DHK, See LC, Liau SB, Tsai RJ . Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol 2000; 84: 973–978.
Shimazaki J, Kosaka K, Shimmura S, Tsutoba K . Amniotic membrane transplantation with conjunctival autograft for recurrent pterygium. Ophthalmology 2003; 110: 119–124.
Chen JK, Tsai RJF, Lin SS . Fibroblasts isolated from human pterygia exhibit transformed cell characteristics. In vitro Cell Dev Biol 1994; 30A: 243–248.
Solomon A, Li DQ, Lee SB, Tseng SCG . Regulation of collagenase, stromelysin, urokinase-type plasminogen activation in primary pterygium fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci 2000; 41: 2154–2163.
Sippel KC, Ma JJK, Foster CS . Amniotic membrane surgery. Curr Opin Ophthalmol 2001; 12: 269–281.
Fukuda K, Chikama T, Nakamura M, Nishida T . Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea 1999; 18: 73–79.
Terranova VP, Lyall RM . Chemotaxis of human gingival epithelial cells to laminin. A mechanism for epithelial cell apical migration. J Periodontol 1986; 57: 311–317.
Khodadoust AA, Silverstein AM, Kenyon DR, Dowling JE . Adhesion of regenerating corneal epithelium. The role of basement membrane. Am J Ophthalmol 1968; 65: 339–348.
Guo M, Grinnell F . Basement membrane and human epidermal differentiation in vitro. J Invest Dermatol 1989; 93: 372–378.
Meller D, Tseng SCG . Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci 1999; 40: 876–886.
Kruse FE, Rohrschneider K, Volcker HE . Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology 1999; 106: 1504–1511.
Chen HJ, Pires RT, Tseng SC . Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol 2000; 84: 826–833.
Choi TH, Tseng SC . In vivo and in vitro demonstration of epithelial cell induced myofibroblast differentiation of keratocytes and an inhibitory effect by amniotic membrane. Cornea 2001; 20: 197–204.
Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC . Suppression of TGF beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res 2000; 20: 325–334.
Koizumi N, Fullwood NJ, Bairaktaris G, Inatomi T, Kinoshita S, Quantock AJ . Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci 2000; 41: 2506–2513.
Ti S, Tseng SCG . Management of primary and recurrent pterygium using amniotic membrane transplantation. Curr Opin Ophthalmol 2002; 13: 204–212.
Lewallen S . A randomized trial of conjunctival autografting for pterygium in the tropics. Ophthalmology 1989; 96: 1612–1614.
McLean C . Pterygium excision with conjunctival autografting. Br J Ophthalmol 1994; 78: 421.
Singh G, Wilson MR, Foster CS . Long-term follow-up study of mitomycin eye drops as adjunctive treatment for pterygia and its comparison with conjunctival autograft transplantation. Cornea 1990; 9: 331–334.
Hirst LW, Sebban A, Chant D . Pterygium recurrence time. Ophthalmology 1994; 101: 755–758.
Acknowledgements
This work was supported by a grant from the National Institute of Health (NIH), Bethesda, MD, EY00412-03 (RSC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Approval for conducting this project was obtained from the Institutional Review Board of University of California, Irvine (HS# 2003-3126)
Rights and permissions
About this article
Cite this article
Memarzadeh, F., Fahd, A., Shamie, N. et al. Comparison of de-epithelialized amniotic membrane transplantation and conjunctival autograft after primary pterygium excision. Eye 22, 107–112 (2008). https://doi.org/10.1038/sj.eye.6702453
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702453
Keywords
This article is cited by
-
Comparison of conjunctival autograft transplantation and amniotic membrane transplantation for pterygium: a meta-analysis
Graefe's Archive for Clinical and Experimental Ophthalmology (2012)