Abstract
Purpose To assess the effect of diagnostic mydriasis with 1% cyclopentolate on the intraocular pressure (IOP) of patients attending glaucoma, medical retina and cataract clinics.
Methods Levels of agreement for IOP assessment were determined and 95% of repeated readings found to be within ±2 mmHg. The IOP of 83 cataract, 87 medical retinal and 100 glaucoma patients was measured with Goldmann applanation tonometry before and 45 min after dilatation with 1% cyclopentolate. Those showing a substantial (>10 mmHg) increase in IOP underwent gonioscopy to determine if their angles remained open and were medically treated to lower their IOP.
Results An approximately normal distribution of change in IOP following dilatation was seen in all three groups (mean change 0.4 mmHg (95% CI 0.1–0.8)). The proportion of patients with a rise of 5 mmHg or more in the right eye was 7% (95% CI 4–10%). Logistic regression using all right eyes, looking at age, sex, diagnosis, ethnicity, ocular medication, iris colour and lens status (phakic/pseudophakic/aphakic) as risk factors for a rise of IOP of 5 mmHg or more did not reveal any significant contribution. Correlation between results obtained for right and left eyes in the glaucoma group was lower (0.43) than for the other groups (0.66 and 0.72), but the extent to which the direction of change in one eye predicted that in the other was shown to be high. Two glaucoma patients with open angles developed a clinically important (>10 mmHg) sustained rise in IOP requiring treatment.
Conclusions Individual variability in the effects of cyclopentolate on aqueous dynamics may account for the approximately normal distribution of IOP seen following dilatation in all three groups. This variation was in excess of that due to observation error alone. It is recommended that the IOP be rechecked after dilation in glaucoma patients with significantly damaged optic nerve heads. In medical retina and cataract patients, sustained clinically important rises in intraocular pressure following dilation seem rarer.
Similar content being viewed by others
Introduction
Cyclopentolate drops were introduced into clinical practice in 1951 and today are regularly used to dilate pupils in patients presenting to ophthalmology clinics for assessment and follow-up of a wide variety of ophthalmic conditions. Cyclopentolate is a parasympatholytic agent with anti-muscarinic activity, and hence causes pupillary dilatation followed by paralysis of the ciliary muscle. Following the administration of one drop of 1% cyclopentolate, maximum mydriasis occurs in 20–30 min in those with lightly pigmented irides and 30–60 min in those with heavily pigmented irides. Maximum cycloplegia is found to occur in 30–60 min.1
It is well recognised that cycloplegic agents can cause a significant rise in intraocular pressure in susceptible patients. With the advent of gonioscopy it was found that a narrow angle was a crucial factor in predisposing to acute IOP elevation.2 More recently, significant rises in IOP have been found to occur in susceptible eyes without a gonioscopically detectable narrow angle.3 Galin found that in these patients a decrease in aqueous outflow occurred simultaneously with pressure elevation.4 Further studies however have indicated that change in aqueous inflow may be a more crucial factor in determining acute pressure elevations.5
Cycloplegics have been shown to cause significant IOP elevation in only 2% of the apparently normal population, increasing to 23% of patients with known primary open angle glaucoma.1,6 We aimed to assess the effect of diagnostic mydriasis with cyclopentolate on the IOP of patients with a range of different ophthalmic conditions, presenting to routine glaucoma, medical retina and cataract clinics. The possible implications of routinely dilating such patients in this clinical setting are clearly relevant to everyday practice.
Patients and methods
A total of 270 patients attending specialist clinics under Moorfields Eye Hospital were studied, comprising 100 from the glaucoma service, 87 from medical retina and 83 from the cataract service. The patient’s age, sex, ethnic origin, iris colour, presenting problems and current medication (both ocular and systemic) were recorded. Those patients with known narrow angles and anterior chamber pathology were excluded from the study. The diagnostic mix in each patient subgroup is given in Table 1.
The age of our patients ranged from 20–94 years (mean 67.8 years), with 124 male and 146 female patients. Blue irides were present in 152 patients and brown in 115, while three patients had irides of another colour. One hundred and seventy-four patients in the study were of Caucasian origin, 71 Asian, 12 African, 11 Caribbean and two of ethnic origin other than these. There was no major difference in the distribution of patient characteristics between groups.
Intraocular pressure was measured in both eyes in all subjects before administering one drop of 1% cyclopentolate to the conjunctival sac of each eye, with a further IOP check after 45 min. If the IOP was above 25 mmHg at 45 min it was measured again an hour later. Those patients with rises in IOP of greater than 10 mmHg from baseline underwent gonioscopy to determine whether their angle remained open. They were treated with Diamox and their pressure re-checked hourly until it began to fall.
Goldmann applanation tonometry was used to measure IOP. All but 10 readings were taken by a single observer (JH) after training and validation. Interobserver studies with trained individuals prior to the commencement of the study showed over 95% of readings to be within ± 2 mmHg agreement. The remaining 10 individuals in the study had IOP readings by similarly validated individuals (DP, IM).
Results
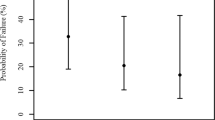
The change in IOP approximated to a normal distribution. There was an overall mean change of 0.4 mmHg (95% CI 0.1–0.8) 45 min after administration of cyclopentolate. The mean change was 0.2 (95% CI −0.4 to +0.8) for glaucoma, 0.4 (95% CI −0.2 to +1.0) for cataract and 0.8 (95% CI 0.1–1.4) for retinal patients (all means given for right eyes only, Figures 1–3). There was no statistically significant difference in distributions between patient groups (two-tailed, unpaired t-test). Similarly the proportion with a pressure rise following dilatation was approximately equal in all groups (Table 2).
The proportion of patients with a rise of 5 mmHg or more in the right eye was 7% (95% CI 4–10%). For glaucoma patients it was 5% (95% CI 1–9%), retinal patients 10% (95% CI 4–17%) and cataract patients 6% (95% CI 1–11%). Four patients had a rise in IOP to a value greater than 25 mmHg. In two of these individuals (one with ocular hypertension, the other with a diagnosis of cataract), the pressure was reducing after a further 1 h with no medical intervention. The remaining two required medical intervention. One patient was a 72-year-old lady with pseudoexfoliative glaucoma on topical Betagan (Levobunolol) and Pilocarpine therapy to both eyes. Initial IOP was 20 mmHg right eye (RE) and 16 mmHg left eye (LE), which increased to 35 mmHg RE and decreased to 10 mmHg LE 45 min after dilatation. The second, a 72-year-old man, had primary open angle glaucoma and was on topical Trusopt (Dorzolamide) therapy to both eyes. His initial IOP was 18 mmHg RE and 20 mmHg LE increasing to 19 mmHg RE and 26 mmHg LE 45 min after dilatation. After a further hour, however, they had risen to 35 mmHg RE and 36 mmHg LE and medical therapy was given. Gonioscopy was undertaken on both patients during the period of raised pressure and revealed wide open drainage angles in each case.
Logistic regression using all right eyes, looking at age, sex, diagnosis, ethnicity, ocular medication, iris colour and lens status (phakic/pseudophakic/aphakic) as risk factors for a rise of IOP of 5 mmHg or more did not reveal any significant contribution.
The correlation between right and left eyes for change in IOP was found to be 0.43 for the glaucoma patients, 0.66 for the medical retinal patients and 0.72 for the cataract patients. The extent to which the direction of change in IOP in one eye predicted the direction of change in the other eye is shown in Table 2. Random allocation of change to two observations would suggest that 10% should not change, 40% should change in the same direction and 50% should change in the opposite direction. The findings in this study show a strong association between results for fellow eyes (chi-squared = 36.5–49.5 P < 0.001). In other words if the intraocular pressure rises following dilation in one eye then it is highly likely to rise in the other eye.
Discussion
In our study we found the changes in IOP to approximate to a normal distribution in all three patient groups studied. Other studies have demonstrated a degree of variation in IOP rise in susceptible eyes but not this normal distribution.
Accuracy of Goldmann tonometry is crucial in assessing what variation in IOP is attributable to dilatation. We established our interobserver variation as being at least 95% of readings within ±2 mmHg. This is well within expected tolerance from previous publications.7,8,9,10 Taking the most conservative estimate, a maximum of 5% of observations might have been expected to exceed ±2 mmHg. Our observation was that 33% of observations changed more than 2 mmHg. Diurnal variation is unlikely to account for such an increase in variation over 45 min hence we believe the most likely cause for the variation to be cyclopentolate administration.
Cycloplegic agents can cause a rise in IOP which may be related to decreased aqueous outflow, resulting from decreased pull on the trabecular meshwork due to ciliary muscle paralysis.3,11 Valle noted an increase in aqueous inflow in patients who experienced a rise in IOP following dilation and in addition suggested a decrease in aqueous outflow in the same patients.5 For many patients in his study, aqueous inflow decreased to a greater extent than aqueous outflow with a resultant fall in IOP. It may well be that the variations in IOP seen in our study represent a normal variation in how inflow and outflow of aqueous is affected by cyclopentolate. This variation does not seem to be affected by the diagnostic category in our study although we did find the correlation between eyes to be less in glaucoma patients (0.43) than for the other patient groups (medical retinal patients 0.66, cataract patients 0.72). Alternative mydriatic agents such as tropicamide or phenyl-epherine may have different effects on intraocular pressure. Our study was limited to one concerntration of a single agent.
We found that the direction of pressure change in one eye strongly predicted the direction of change for the other eye in all groups. This suggests a within person response to topical dilation with ocular dynamics being similar in right and left eyes of the same individual. Although not surprising, to our knowledge, this has not been illustrated in this fashion previously and is clearly of importance for researchers investigating intraocular pressure change.
Two patients in our study showed a sharp increase in IOP following administration of cyclopentolate. Both had a diagnosis of glaucoma and hence were theoretically at increased risk of harm from a pressure rise. One patient with a diagnosis of POAG showed a maximum pressure rise of 12 mmHg in the right eye and 16 mmHg in the left eye at 1 h 45 min (Initial IOP 18 mmHg RE, 20 mmHg LE). This is inconsistent with studies which have reported that the rise in IOP reaches its peak at 45 min post dilatation.1 The relative delay in IOP peak seen in our patient might be due to the Trusopt (Dorzolamide) drops he was using, or an abnormality with aqueous inflow and outflow dynamics related to his POAG. Equally our chosen time period of re-examination may not be the time of peak pressure change. Only a further study would answer this question. The second patient experienced a maximum pressure rise of 17 mmHg in the right eye and a fall of 6 mmHg in the left eye (Initial IOP 20 mmHg RE, 16 mmHg LE). It is not possible for this study to adequately explain this discrepancy. We have demonstrated that there is poorer correlation between right and left eyes in glaucoma patients (although generally the behaviour of one eye predicts the behaviour of the other) and have speculated that topical ocular hypotensive agents may have a part to play in this. The above patient was known to have pseudoexfoliative glaucoma, which may further alter the predictability of response between eyes. Only a further study, examining the variability of response in patients with pseudoexfoliative glaucoma compared to those with POAG would help to answer this.
It was demonstrated by Kronfeld that in certain eyes, cycloplegics can cause a significant rise in IOP with open angles.11 In later studies Harris demonstrated that approximately two out of 100 patients with POAG had a significant rise in IOP following administration of cycloplegics.1 It seems clear therefore, from our study and others, that a small number of patients undergoing routine dilatation with cyclopentolate will experience a significant rise in IOP. It is not routine practice to re-check IOP following dilatation in outpatient clinics. As these patients have open angles and there is no factor identified to predict in whom this is likely to occur, these cases will go undetected. In this study the patients with significant rises in IOP were treated immediately to decrease the risk of any chance of damage to the optic nerve. Other studies have suggested that if untreated the rise in IOP is likely to be sustained for between 4 and 6 h.1
Does this undetected rise in IOP in some patients represent a clinically significant risk of damage to the optic nerve, and if so, should we routinely re-check IOP following administration of cycloplegics? In eyes that have no history of glaucoma it is likely that this undetected rise will have little clinical significance. In eyes already compromised by raised IOP, a further significant rise, for what could be up to 6 h may have detrimental effects. It is therefore recommended that patients known to have glaucoma with severely compromised optic nerve heads have their IOPs rechecked after administration of cycloplegics. An alternative may be the administration of ocular hypotensive agents such as iopidine concurrently with mydriasis. This would need trial to assess its effectivity.
In summary, our study showed an approximately normal distribution in variation of IOP in all three groups studied after the administration of cyclopentolate. In keeping with other studies, we also demonstrated that a few patients with open angles are subject to large pressure increases following cyclopentolate administration. There appears to be no predictive factor for such patients who may be susceptible to such an IOP rise. In an eye already compromised by glaucoma, any sustained rise in IOP may result in further clinically significant damage to ganglion cells. For this reason it is recommended that the IOP is rechecked in all glaucoma patients with significant optic nerve damage following administration of a cycloplegic. The potential for harm to patients attending medical retinal and cataract clinics appears to be much lower with no clear indication of a need to routinely recheck the IOP in these groups.
References
Harris LS . Cycloplegic induced intraocular pressure elevations. Arch Ophthalmol 1968; 79: 242–246
Harris LS, Galin MA, Thomas D, Mittag W . Cycloplegic provocation testing after topical administration with steroids. Arch Ophthalmol 1971; 86: 12–14
Velasco Cabreva J, Euroa Mozos P, Garcia Sanchez J, Bermudez Rodriguez F . Changes in intraocular pressure due to cycloplegia. CLAO J 1998; 86: 111–114
Galin MA . The mydriasis provocation test. Arch Ophthalmol 1961; 66: 353–355
Valle O . Effect of cyclopentolate on the aqueous dynamics in incipient or suspected open-angle glaucoma. Acta Ophthalmol 1973; XXI: 52–59
Portney GL, Purcell TW . The influence of tropicamide on intraocular pressure. Ann Ophthalmol 1975; 7: 31–34
Thornbury W . The accuracy of clinical applanation tonometry. Acta Ophthalmol 1978; 56: 1–5
Phelps CD, Phelps GK . Measurement of intraocular pressure: a study of its reproducibility. Graefes Arch Clin Exp Ophthalmol 1976; 198: 39–43
Sudesh S, Moseley MJ, Thompson JR . Accuracy of Goldmann tonometry in clinical practice. Acta Ophthalmol 1993; 71: 185–188
Dielemans I, Vingerling JR, Hofman A, Grobbee DE, de Jong PT . Reliabilty of intraocular pressure measurement with Goldmann applanation tonometry in epidemiological studies. Graefes Arch Clin Exp Ophthalmol 1994; 232: 141–144
Kronfeld P, McGarry HI, Smith HF . The effect of mydriatics upon intraocular pressure in primary wide angle glaucoma. Am J Ophthalmol 1943; 26: 245
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hancox, J., Murdoch, I. & Parmar, D. Changes in intraocular pressure following diagnostic mydriasis with cyclopentolate 1%. Eye 16, 562–566 (2002). https://doi.org/10.1038/sj.eye.6700146
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6700146
Keywords
This article is cited by
-
Risk of acute angle-closure and changes in intraocular pressure after pupillary dilation in patients with diabetes
Eye (2023)
-
The change in intraocular pressure after pupillary dilation in eyes with pseudoexfoliation glaucoma, primary open angle glaucoma, and eyes of normal subjects
International Ophthalmology (2015)