Abstract
Background/objectives:
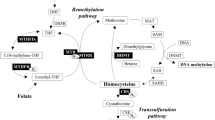
Choline is an essential nutrient involved in one-carbon metabolism, but its role in mechanisms underlying meiotic non-disjunction is poorly known. The relationship between folate-homocysteine metabolic pathway gene polymorphism and Down syndrome (DS) risk has been widely analyzed, but there are limited reports on its correlation with choline metabolism. In the present case–control association study, we investigated the relationship of three single-nucleotide polymorphisms (SNPs) (phosphatidylethanolamine N-methyltransferase (PEMT) rs12325817, choline dehydrogenase (CHDH) rs12676 and homocysteine methyltransferase (BHMT) rs3733890) of choline metabolism with risk for DS.
Subject/methods:
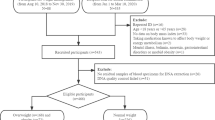
Genotyping of 228 mothers of a down syndrome child (DSM) and 200 control mothers (CMs) for all SNPs was performed by PCR coupled with restriction fragment length polymorphism method.
Results:
A significantly increased risk for BHMT +742AA genotype with an odds ratio of 4.96 (95% confidence interval (CI): 1.66–14.88, P=0.0036) was observed. For PEMT rs12325817 and CHDH rs12676, no significant difference in allelic and genotypic frequencies was observed. In genotypic combination analysis considering PEMT −744GG/CHDH +432GG/BHMT +742GG as the reference combination, PEMT −744GC/CHDH +432GG/BHMT +742GG genotypic combination was significantly higher in DSM compared with that in CMs with an odds ratio of 2.061 (95% CI: 1.10–3.86, P=0.0342). We also observed an epistatic interaction between methylenetetrahydrofolate reductase (MTHFR) rs1801133 and choline metabolic pathway gene variants.
Conclusions:
Our findings indicate impaired choline metabolism showing a greater risk for DS, especially in a population associated with homocysteine-folate impairment. Further studies are required to confirm our findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lamb NE, Freeman SB, Savage-Austin A, Pettay D, Taft L, Hersey J et al. Susceptible chiasmata configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat Genet 1996; 14: 400–405.
Morris JK, Mutton DE, Alberman E . Revised estimates of the maternal age specific live birth prevalence of Down’s syndrome. J Med Screen 2002; 9: 2–6.
James SJ, Pogribna M, Pogribny IP, Melnyk S, Hine RJ, Gibson JB et al. Abnormal folate metabolism and mutation in the methylenetetrahydrofolate reductase gene may be maternal risk factors for Down syndrome. Am J Clin Nutr 1999; 70: 495–501.
Rai AK, Singh S, Mehta S, Kumar A, Pandey LK, Raman R et al. MTHFR C677T and A1298C are risk factors for Down syndrome in Indian mothers. J Hum Genet 2006; 51: 278–283.
Sukla KK, Jaiswal SK, Rai AK, Mishra OP, Gupta V, Kumar A et al. Role of folate-homocysteine pathway gene polymorphisms and Nutritional cofactors in Down syndrome: a triad study. Hum Reprod 2015; 30: 1982–1993.
Zeisel SH, Blusztajn JK . Choline and human nutrition. Annu Rev Nutr 1994; 14: 269–296.
Olthof MR, vanVliet T, Boelsma E, Verhoef P . Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr 2003; 133: 4135–4138.
Bailey LB, Gregory JF . Folate metabolism and requirements. J Nutr 3rd 1999; 129: 779–782.
Sunden S, Renduchintala M, Park E, Miklasz S, Garrow T . Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch Biochem Biophys 1997; 345: 171–174.
Millan NS, Garrow TA . Human betaine-homocysteine methyltransferase is a zinc metalloenzyme. Arch Biochem Biophys 1998; 356: 93–98.
Da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH . Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J 2006; 20: 1336–1344.
Weisberg IS, Park E, Ballman KV, Berger P, Nunn M, Suh DS et al. Investigations of a common genetic variant in betaine-homocysteine methyltransferase (BHMT in coronary artery disease. Atherosclerosis 2003; 167: 205–214.
Sukla KK, Raman R . Association of MTHFR and RFC1 gene polymorphism with hyperhomocysteinemia and its modulation by vitamin B12 and folic acid in an Indian population. Eur J Clin Nutr 2012; 66: 111–118.
Jaiswal SK, Sukla KK, Kumari N, Lakhotia AR, Kumar A, Rai AK . Promoter polymorphisms of DNMT3B gene and risk for Down syndrome: a case–control study. Birth Defects Res (PART A) 2015; 103: 298–304.
Miller SA, Dykes DD, Polesky HF . A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215.
Ananth CV, Elsasser DA, Kinzler WL, Peltier MR, Getahun D, Leclerc D et al. Polymorphisms in methionine synthase associations with colorectal cancer. Cancer Epidemiol Biomarkers Prev 2007; 15: 2408–2417.
Xu X, Gammon MD, Zeisel SH, Lee YL, Wetmur JG, Teitelbaum SL et al. Choline metabolism and risk of breast cancer in a population-based study. FASEB J 2008; 22: 2045–2052.
Kumar J, Das SK, Sharma P, Karthikeyan G, Ramakrishnan L, Sengupta S . Homocysteine levels are associated with MTHFR A1298C polymorphism in Indian population. J Hum Genet 2005; 50: 655–663.
Institute of Medicine, and National Academy of Sciences USA. Dietary Reference Intakes for Folate, Thiamin, Riboflavin, Niacin, Vitamin B12, Panthothenic Acid, Biotin, and Choline, vol. 1 National Academy Press: Washington, DC, USA, 1998.
Shaw GM, Lu W, Zhu H, Yang W, Briggs FB, Carmichael SL et al. 118 SNPs of folate related genes and risks of spina bifida and conotruncal heart defects. BMC Med Genet 2009; 10: 49.
Mills JL, Carter TC, Kay DM, Browne ML, Brody LC, Liu A et al. Folate and vitamin B12-related genes and risk for omphalocele. Hum Genet 2012; 131: 739–746.
Hazra A, Wu K, Kraft P, Fuchs CS, Giovannucci EL, Hunter DJ . Twenty-four non-synonymous polymorphisms in the one-carbon metabolic pathway and risk of colorectal adenoma in the Nurses’ Health Study. Carcinogenesis 2007; 28: 1510–1519.
Morin I, Platt R, Weisberg I, Sabbaghian N, Wu Q, Garrow TA et al. Common variant in betaine-homocysteine methyltransferase (BHMT and risk for spina bifida. Am J Med Genet 2003; 119A: 172–173.
Mostowska A, Hozyasz KK, Wojcicki P, Dziegelewska M, Jagodzinski PP . Associations of folate and choline metabolism gene polymorphisms with orofacial clefts. J Med Genet 2010; 47: 809–815.
Mostowska A, Myka M, Lianeri M, Roszak A, Jagodziński PP . Folate and choline metabolism gene variants and development of uterine cervical carcinoma. Clin Biochem 2011; 44: 596–600.
Hozyasz KK, Mostowska A, Szaflarska-Poplawska A, Lianeri M, Jagodzinski PP . Polymorphic variants of genes involved in homocysteine metabolism in celiac disease. Mol Biol Rep 2012; 39: 3123–3130.
Popp RA, Farcas MF, Trifa AP, Crisan TO, Militaru MS, Pop IV . Association of betaine-homocysteine S-methyltransferase gene G742A SNP and male infertility. Rev Rom Med Labor 2012; 20: 57–62.
Ying H, ErJun C, Yue M, JinLu L, RenJi C . BHMT gene polymorphisms as risk factors for cleft lip and cleft palate in a Chinese population. Biomed Environ Sci 2011; 24: 89–93.
Heil SG, Lievers KJ, Boers GH, Verhoef P, den Heijer M, Trijbels FJ et al. Betaine homocysteine methyltransferase (BHMT: genomic sequencing and relevance to hyperhomocysteinemia and vascular disease in humans. Mol. Genet Metab 2000; 71: 511–519.
Zampieri BL, Biselli JM, Goloni-Bertollo EM, Pavarino EC . BHMT G742A and MTHFD1 G1958A polymorphisms and Down syndrome risk in the Brazilian population. Genet Test Mol Biomarkers 2012; 16: 628–631.
Zampieri BL, Biselli JM, Goloni-Bertollo EM, Vannucchi H, Carvalho VM, Cordeiro JA et al. Maternal risk for Down syndrome is modulated by genes involved in folate metabolism. Disease Markers 2012; 32: 73–81.
Amorim MR, Moura CM, Gomes AD . Betaine-homocysteine methyltransferase 742 g> a polymorphism and risk of down syndrome offspring in a Brazilian population. Mol Biol Rep 2013; 40: 4685–4689.
Koushik A, Kraft P, Fuchs CS, Hankinson SE, Willett WC, Giovannucci EL et al. Nonsynonymous polymorphisms in genes in the one-carbon metabolism pathway and reductase and betaine-homocysteine S-methyltransferase genes: risk of placental abruption. Mol Genet Metab 2006; 91: 104–110.
Boyles AL, Billups AV, Deak KL, Siegel DG, Mehltretter L, Slifer SH et al. Neural tube defects and folate pathway genes: family-based association tests of gene–gene and gene–environment interactions. Environ Health Perspect 2006; 114: 1547–1552.
Singh PR, Lele SS, Mukherjee MS . Gene polymorphisms and low dietary intake of micronutrients in coronary artery disease. J Nutrigenet Nutrigenom 2011; 4: 203–209.
Zhu H, Curry S, Wen S, Wicker NJ, Shaw GM, Lammer EJ et al. Are the betaine-homocysteine methyltransferase (BHMT and BHMT2) genes risk factors for spina bifida and orofacial clefts? Am J Med Genet 2005; 135A: 274–277.
Giusti B, Saracini C, Bolli P, Magi A, Sestini I, Sticchi E et al. Genetic analysis of 56 polymorphisms in 17 genes involved in methionine metabolism in patients with abdominal aortic aneurysm. J Med Genet 2008; 45: 721–730.
Szczepańska M, Mostowska A, Wirstlein P, Lianeri M, Marianowski P, Skrzypczak J et al. Polymorphic variants of folate and choline metabolism genes and the risk of endometriosis-associated infertility. Eur J Obstet Gynecol Reprod Biol 2011; 157: 67–72.
Boyles AL, Wilcox AJ, Taylor JA, Shi M, Weinberg CR, Meyer K et al. Oral-facial clefts and gene polymorphisms in the metabolism of folate/one-carbon and vitamin A: a pathway-wide association study. Genet Epidemiol 2009; 33: 247–255.
Hu Y, Chen E, Mu Y, Li J, Chen R . BHMT gene polymorphisms as risk factors for cleft lip and cleft palate in a Chinese population. Biomed Environ Sci 2011; 24: 89–93.
Pawlik P, Mostowska A, Lianeri M, Sajdak S, Ke˛dzia H, Jagodzinski PP . Folate and choline metabolism gene variants in relation to ovarian cancer risk in the Polish population. Mol Biol Rep 2012; 39: 5553–5560.
Li F, Feng Q, Lee C, Wang S, Pelleymounter LL, Moon I et al. Human betaine-homocysteine methyltransferase (BHMT and BHMT2: common gene sequence variation and functional characterization. Mol Genet Metab 2008; 94: 326–335.
Acknowledgements
We thank Down syndrome mothers and control mothers for participation in the study. We record words of gratitude to our teacher Professor Rajiva Raman for critical analysis and comments. We are thankful to Professor SK Singh, Institute of Medical Sciences, for permitting us to conduct chemiluminescence assay of vitamin B12 and folic acid in their lab. We also thank UGC-sponsored Centre for Advanced Study, Department of Zoology and Interdisciplinary School of Life Sciences, BHU, Varanasi for HPLC analysis of homocysteine, and Centre for Genetic Disorders, BHU for chromosomal analysis. We also acknowledge Ms Radha Raghuraman, Graduate Student, Synaptic Plasticity and Memory Lab, Department of Physiology and Yong Loo Lin, School of Medicine, National University of Singapore for proofreading the final version of the manuscript. This work forms part of the contribution of the Disease biology thrust area under DBT-sponsored Interdisciplinary School of Life Sciences, BHU. ICMR, New Delhi, is duly acknowledged for fellowship to SKJ.
Author contributions
Conceived and designed the experiments: SKJ, KKS and AKR; performed the experiments: SKJ and AC; analyzed the data: KKS, SKJ and AKR; wrote the manuscript: KKS, SKJ and AKR; grant for the study: AKR; clinical samples provided by AK and ARL; collected the samples: SKJ; gave critical comments on the manuscript: AK and ARL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Jaiswal, S., Sukla, K., Chauhan, A. et al. Choline metabolic pathway gene polymorphisms and risk for Down syndrome: An association study in a population with folate-homocysteine metabolic impairment. Eur J Clin Nutr 71, 45–50 (2017). https://doi.org/10.1038/ejcn.2016.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2016.190