Abstract
Background/objective:
Serum metabolites have been linked to higher risk of chronic diseases but determinants of serum metabolites are not clear. We aimed to investigate the association between habitual diet as a modifiable risk factor and relevant serum metabolites.
Subjects/methods:
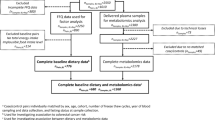
This cross-sectional study comprised 2380 EPIC-Potsdam participants. Intake of 45 food groups was assessed by food frequency questionnaire and concentrations of 127 serum metabolites were measured by targeted metabolomics. Reduced rank regression was used to find dietary patterns that explain the maximum variation of metabolites.
Results:
In the multivariable-adjusted model, the proportion of explained variation by habitual diet was ranked as follows: acyl-alkyl-phosphatidylcholines (5.7%), sphingomyelins (5.1%), diacyl-phosphatidylcholines (4.4%), lyso-phosphatidylcholines (4.1%), acylcarnitines (3.5%), amino acids (2.2%) and hexose (1.6%). A pattern with high intake of butter and low intake of margarine was related to acylcarnitines, acyl-alkyl-phosphatidylcholines, lyso-phosphatidylcholines and hydroxy-sphingomyelins, particularly with saturated and monounsaturated fatty acid side chains. A pattern with high intake of red meat and fish and low intake of whole-grain bread and tea was related to hexose and phosphatidylcholines. A pattern consisting of high intake of potatoes, dairy products and cornflakes particularly explained methionine and branched chain amino acids. Dietary patterns related to type 2 diabetes-relevant metabolites included high intake of red meat and low intake of whole-grain bread, tea, coffee, cake and cookies, canned fruits and fish.
Conclusions:
Dietary patterns characterized by intakes of red meat, whole-grain bread, tea and coffee were linked to relevant metabolites and could be potential targets for chronic disease prevention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 2009; 139: 1073–1081.
Suhre K, Meisinger C, Doring A, Altmaier E, Belcredi P, Gieger C et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One 2010; 5: e13953.
Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol 2012; 8: 615.
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009; 9: 311–326.
Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH . Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One 2010; 5: e15234.
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17: 448–453.
Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013; 62: 639–648.
Gibney MJ, Walsh M, Brennan L, Roche HM, German B, van Ommen B . Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr 2005; 82: 497–503.
Primrose S, Draper J, Elsom R, Kirkpatrick V, Mathers JC, Seal C et al. Metabolomics and human nutrition. Br J Nutr. 2011; 105: 1277–1283.
Hyotylainen T, Bondia-Pons I, Oresic M . Lipidomics in nutrition and food research. Mol Nutr Food Res 2013; 57: 1306–1318.
Altmaier E, Kastenmuller G, Romisch-Margl W, Thorand B, Weinberger KM, Illig T et al. Questionnaire-based self-reported nutrition habits associate with serum metabolism as revealed by quantitative targeted metabolomics. Eur J Epidemiol 2011; 26: 145–156.
Hoffmann K, Schulze MB, Schienkiewitz A, Nothlings U, Boeing H . Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol 2004; 159: 935–944.
Boeing H, Korfmann A, Bergmann MM . Recruitment procedures of EPIC-Germany. European Investigation into Cancer and Nutrition. Ann Nutr Metab 1999; 43: 205–215.
Boeing H, Wahrendorf J, Becker N . EPIC-Germany—a source for studies into diet and risk of chronic diseases. European Investigation into Cancer and Nutrition. Ann Nutr Metab 1999; 43: 195–204.
Ford ES, Schulze MB, Bergmann MM, Thamer C, Joost HG, Boeing H . Liver enzymes and incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes Care 2008; 31: 1138–1143.
Kroke A, Klipstein-Grobusch K, Voss S, Moseneder J, Thielecke F, Noack R et al. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr 1999; 70: 439–447.
Boeing H, Bohlscheid-Thomas S, Voss S, Schneeweiss S, Wahrendorf J . The relative validity of vitamin intakes derived from a food frequency questionnaire compared to 24-hour recalls and biological measurements: results from the EPIC pilot study in Germany. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997; 26 (Suppl 1), S82–S90.
Bohlscheid-Thomas S, Hoting I, Boeing H, Wahrendorf J . Reproducibility and relative validity of energy and macronutrient intake of a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997; 26 (Suppl 1), S71–S81.
Bohlscheid-Thomas S, Hoting I, Boeing H, Wahrendorf J . Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997; 26 (Suppl 1), S59–S70.
Romisch-Margl W, Prehn C, Bogumil R, Rohring C, Suhre K, Adamski J . Procedure for tissue sample preparation and metabolite extraction for high-throughput targeted metabolomics. Metabolomics 2012; 8: 133–142.
US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). Guidance for industry. Bioanalytical Method Validation 2001.
Floegel A, Drogan D, Wang-Sattler R, Prehn C, Illig T, Adamski J et al. Reliability of serum metabolite concentrations over a 4-month period using a targeted metabolomic approach. PLoS One 2011; 6: e21103.
Schulze MB, Hoffmann K, Kroke A, Boeing H . Dietary patterns and their association with food and nutrient intake in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Br J Nutr 2001; 85: 363–373.
von Ruesten A, Feller S, Bergmann MM, Boeing H . Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr 2013; 67: 412–419.
Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005; 82: 675–684. (quiz 714–5).
Fung TT, Schulze MB, Hu FB, Hankinson SE, Holmes MD . A dietary pattern derived to correlate with estrogens and risk of postmenopausal breast cancer. Breast Cancer Res Treat 2012; 132: 1157–1162.
Meyer J, Doring A, Herder C, Roden M, Koenig W, Thorand B . Dietary patterns, subclinical inflammation, incident coronary heart disease and mortality in middle-aged men from the MONICA/KORA Augsburg cohort study. Eur J Clin Nutr 2011; 65: 800–807.
McNaughton SA, Mishra GD, Brunner EJ . Food patterns associated with blood lipids are predictive of coronary heart disease: the Whitehall II study. Br J Nutr 2009; 102: 619–624.
O'Sullivan A, Gibney MJ, Brennan L . Dietary intake patterns are reflected in metabolomic profiles: potential role in dietary assessment studies. Am J Clin Nutr 2011; 93: 314–321.
Quehenberger O, Dennis EA . The human plasma lipidome. N Engl J Med 2011; 365: 1812–1823.
McBride KL, Belmont JW, O'Brien WE, Amin TJ, Carter S, Lee BH . Heritability of plasma amino acid levels in different nutritional states. Mol Genet Metab 2007; 90: 217–220.
Hers HG . Mechanisms of blood glucose homeostasis. J Inherit Metab Dis 1990; 13: 395–410.
Pekala J, Patkowska-Sokola B, Bodkowski R, Jamroz D, Nowakowski P, Lochynski S et al. L-carnitine—metabolic functions and meaning in humans life. Curr Drug Metab 2011; 12: 667–678.
US Department of Agriculture, Agricultural Research Service. 2012. USDA National Nutrient Database for Standard Reference, Release 25. Nutrient Data Laboratory Homepagehttp://www.ars.usda.gov/ba/bhnrc/ndl (accessed: 14th January 2013).
FAO. Nutritional studies: amino-acid content of foods and biological data on proteins. FAO Nutr Stud 1970; 24: 1–285.
Schonfeldt HC, Gibson Hall N . Dietary protein quality and malnutrition in Africa. Br J Nutr 2012; 108 (Suppl 2), S69–S76.
Zeisel SH . Epigenetic mechanisms for nutrition determinants of later health outcomes. Am J Clin Nutr 2009; 89: 1488S–1493SS.
Fardet A . New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev 2010; 23: 65–134.
van Dam RM, Grievink L, Ocke MC, Feskens EJ . Patterns of food consumption and risk factors for cardiovascular disease in the general Dutch population. Am J Clin Nutr 2003; 77: 1156–1163.
Smith DR, Wood R, Tseng S, Smith SB . Increased beef consumption increases apolipoprotein A-I but not serum cholesterol of mildly hypercholesterolemic men with different levels of habitual beef intake. Exp Biol Med (Maywood) 2002; 227: 266–275.
Murphy KJ, Thomson RL, Coates AM, Buckley JD, Howe PR . Effects of eating fresh lean pork on cardiometabolic health parameters. Nutrients 2012; 4: 711–723.
Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 2011; 94: 1088–1096.
Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S . Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr 2012; 142: 1304–1313.
Jing Y, Han G, Hu Y, Bi Y, Li L, Zhu D . Tea consumption and risk of type 2 diabetes: a meta-analysis of cohort studies. J Gen Intern Med 2009; 24: 557–562.
Cole LK, Vance JE, Vance DE . Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim Biophys Acta 2011; 1821: 754–761.
Li Z, Vance DE . Phosphatidylcholine and choline homeostasis. J Lipid Res 2008; 49: 1187–1194.
Raubenheimer PJ, Nyirenda MJ, Walker BR . A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes 2006; 55: 2015–2020.
Zock PL, Katan MB . Butter, margarine and serum lipoproteins. Atherosclerosis 1997; 131: 7–16.
Wolk A, Vessby B, Ljung H, Barrefors P . Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr 1998; 68: 291–295.
Schatzkin A, Kipnis V . Could exposure assessment problems give us wrong answers to nutrition and cancer questions? J Natl Cancer Inst 2004; 96: 1564–1565.
Acknowledgements
We specially thank Ellen Kohlsdorf and Wolfgang Bernigau for data management. We also thank Werner Römisch-Margl, Julia Scarpa, and Arsin Sabunchi for metabolomics measurements performed at the Helmholtz Centrum München, Genome Analysis Center, Metabolomics Core Facility. We are thankful to all the EPIC-Potsdam study participants for their devoted participation in the study. This study was supported by the Federal Ministry of Science, Germany (grant no. 01 EA 9401), the European Union (grant no. SOC 95 201408 05F02) and the German Cancer Aid (grant no. 70-2201-Bo2), and by a grant from the Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD eV) (Förderkennzeichen: 01G10922).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Contributors: AF designed the research, conducted the data analysis, interpreted the data, wrote the manuscript and had primary responsibility for data integrity and the final content; AvR, DD, MBS contributed to the conception of this study, helped with the interpretation of the results and provided critical comments on the manuscript; CP and JA conducted metabolomics measurements and provided critical comments on the manuscript; TP and HB obtained funding, designed the research, helped with the interpretation of the results and provided critical comments on the manuscript.
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Floegel, A., von Ruesten, A., Drogan, D. et al. Variation of serum metabolites related to habitual diet: a targeted metabolomic approach in EPIC-Potsdam. Eur J Clin Nutr 67, 1100–1108 (2013). https://doi.org/10.1038/ejcn.2013.147
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.147
Keywords
This article is cited by
-
Associations of plasma glycerophospholipid profile with modifiable lifestyles and incident diabetes in middle-aged and older Chinese
Diabetologia (2022)
-
Advances in dietary pattern analysis in nutritional epidemiology
European Journal of Nutrition (2021)
-
Caution in studying and interpreting the lupus metabolome
Arthritis Research & Therapy (2020)
-
Identification of metabolites associated with prostate cancer risk: a nested case-control study with long follow-up in the Northern Sweden Health and Disease Study
BMC Medicine (2020)
-
Untargeted plasma metabolomic profiles associated with overall diet in women from the SU.VI.MAX cohort
European Journal of Nutrition (2020)